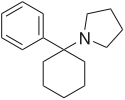
Rolicyclidine
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 2201-39-0 |
| PubChem (CID) | 62436 |
| DrugBank |
DB01549 |
| ChemSpider |
56218 |
| UNII |
183O9O9JE3 |
| ChEBI |
CHEBI:60805 |
| Chemical and physical data | |
| Formula | C16H23N |
| Molar mass | 229.361 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Rolicyclidine (PCPy) is a dissociative anesthetic drug with hallucinogenic and sedative effects. It is similar in effects to phencyclidine but is slightly less potent and has less stimulant effects [1] instead producing a sedative effect described as being somewhat similar to a barbiturate, but with additional PCP-like dissociative, anaesthetic and hallucinogenic effects.[2] Due to its similarity in effects to PCP, PCPy was placed into the Schedule I list of illegal drugs in the 1970s, although it has never been widely abused and is now little known.
See also
References
| Inhalational | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injection |
| ||||||||||||||
| |||||||||||||||
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D1-like |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||||||||||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||
See also: GABAergics • GHBergics • Glycinergics | |||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.