Levosulpiride
 | |
| Names | |
|---|---|
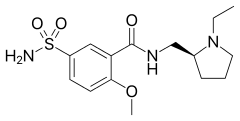
| IUPAC name
N-[[(2S)-(−)-1-ethylpyrrolidin-2-yl]methyl]-2-methoxy-5-sulfamoylbenzamide | |
| Identifiers | |
| 23672-07-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:4119 |
| ChEMBL | ChEMBL267044 |
| ChemSpider | 599749 |
| 958 | |
| KEGG | D07312 |
| PubChem | 688272 |
| UNII | JTG7R315LK |
| |
| |
| Properties | |
| C15H23N3O4S | |
| Molar mass | 341.43 g·mol−1 |
| Pharmacology | |
| N05AL07 (WHO) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Levosulpiride is a substituted benzamide antipsychotic,[1] reported to be a selective antagonist of dopamine D2 receptor activity [2]on both central and peripheral levels. It is an atypical neuroleptic and a prokinetic agent.[2] Levosulpiride is also claimed to have mood elevating properties.
Chemically, it is the (S)-(−)-enantiomer of sulpiride.
Uses
Levosulpiride is used in the treatment of:
- psychoses
- particularly negative symptoms of schizophrenia
- anxiety disorders
- dysthymia
- vertigo
- dyspepsia
- irritable bowel syndrome
- premature ejaculation.
Levosulpiride is not currently licensed for treatment of premature ejaculation in the UK or other European countries.[3]
Side effect
Side effects include amenorrhea, gynecomastia, galactorrhea, changes in libido, and neuroleptic malignant syndrome.[4] In the U.S., as of 2013 only one case of adverse reaction to Levosulpiride had been recorded on the FDA Adverse Event Reporting System Database.[5] A case of rapid onset resistant dystonia (involuntary movements of the trunk, lips, and arms) caused by low dose levosulpiride was reported in India.[6]
Mechanism of action
In contrast to most other neuroleptics which block both dopamine D1 and D2 receptors, sulpiride is more selective and acts primarily as a dopamine D2 antagonist. Sulpiride appears to lack effects on norepinephrine, acetylcholine, serotonin, histamine, or gamma-aminobutyric acid (GABA) receptors. [7]
Pharmacodynamics
Sulpiride is a substituted benzamide derivative and a selective dopamine D2 antagonist with antipsychotic and antidepressant activity. Other benzamide derivatives include metoclopramide, tiapride, and sultopride.[8]
References
- ↑ Generon. "Levosulpiride - S-(-)-Sulpiride - >98% Generon". www.generon.co.uk. Retrieved 2016-08-31.
- 1 2 http://www.stratech.co.uk/. "Levosulpiride | Stratech Scientific Ltd". www.stratech.co.uk. Retrieved 2016-08-31.
- ↑ Poluzzi, Elisabetta; Raschi, Emanuel; Koci, Ariola; Moretti, Ugo; Spina, Edoardo; Behr, Elijah R.; Sturkenboom, Miriam; Ponti, Fabrizio De (2013-04-04). "Antipsychotics and Torsadogenic Risk: Signals Emerging from the US FDA Adverse Event Reporting System Database". Drug Safety. 36 (6): 467–479. doi:10.1007/s40264-013-0032-z. ISSN 0114-5916. PMC 3664739
 . PMID 23553446.
. PMID 23553446. - ↑ http://www.drugsupdate.com/generic/view/860
- ↑ Poluzzi, Elisabetta; Raschi, Emanuel; Koci, Ariola; Moretti, Ugo; Spina, Edoardo; Behr, Elijah R.; Sturkenboom, Miriam; Ponti, Fabrizio De (2013-04-04). "Antipsychotics and Torsadogenic Risk: Signals Emerging from the US FDA Adverse Event Reporting System Database". Drug Safety. 36 (6): 467–479. doi:10.1007/s40264-013-0032-z. ISSN 0114-5916. PMC 3664739
 . PMID 23553446.
. PMID 23553446. - ↑ "Rapid onset resistant dystonia with low dose of Levosulpiride". 2016-08-24.
- ↑ http://www.drugbank.ca/drugs/DB00391
- ↑ http://www.drugbank.ca/drugs/DB00391