Spermidine
| | |
 | |
| Identifiers | |
|---|---|
| 124-20-9 | |
| 3D model (Jmol) | Interactive image |
| 3DMet | B01214 |
| 1698591 | |
| ChEBI | CHEBI:16610 |
| ChEMBL | ChEMBL19612 |
| ChemSpider | 1071 |
| DrugBank | DB03566 |
| ECHA InfoCard | 100.004.264 |
| EC Number | 204-689-0 |
| 454510 | |
| 2390 | |
| KEGG | C00315 |
| MeSH | Spermidine |
| PubChem | 1102 |
| RTECS number | EJ7000000 |
| UN number | 2735 |
| |
| |
| Properties | |
| C7H19N3 | |
| Molar mass | 145.25 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Ichtyal, ammoniacal |
| Density | 925 mg mL−1 |
| Melting point | 22 to 25 °C (72 to 77 °F; 295 to 298 K) |
| 145 g L−1 (at 20 °C) | |
| log P | −0.504 |
| UV-vis (λmax) | 260 nm |
| Absorbance | 0.1 |
| Refractive index (nD) |
1.479 |
| Hazards | |
| GHS pictograms |  |
| GHS signal word | DANGER |
| H314 | |
| P280, P305+351+338, P310 | |
| EU classification (DSD) |
|
| R-phrases | R34 |
| S-phrases | S26, S36/37/39 |
| Flash point | 112 °C (234 °F; 385 K) |
| Related compounds | |
| Related amines |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Spermidine is a polyamine compound (C
7H
19N
3) found in ribosomes and living tissues, and having various metabolic functions within organisms. It was originally isolated from semen.[1]
Function
Polyamines, such as spermidine, are polycationic aliphatic amines and are multifunctional. They serve vital roles in cell survival.
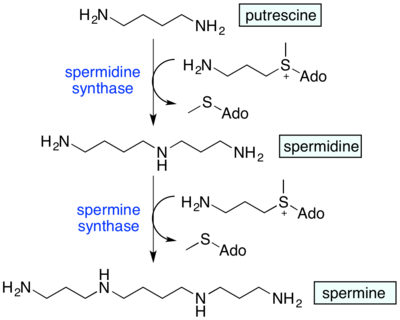
Spermidine synthase (SPDS) catalyzes the formation of spermidine from putrescine. Spermidine is a precursor to further polyamines, such as spermine and its structural isomer thermospermine.
Spermidine synchronizes an array of biological processes (such as Ca2+, Na+, K+ -ATPase) thus maintaining membrane potential and controlling intracellular pH and volume. Spermidine regulates biological processes, such as Ca2+ influx by glutamatergic N-methyl-d-aspartate receptor (NMDA receptor), which has been associated with nitric oxide synthase (NOS) and cGMP/PKG pathway activation and a decrease of Na+,K+-ATPase activity in cerebral cortex synaptosomes.
Spermidine is a longevity agent in mammals due to various mechanisms of action, which are just beginning to be understood. Autophagy is the main mechanism at the molecular level, but evidence has been found for other mechanisms, including inflammation reduction, lipid metabolism, and regulation of cell growth, proliferation and death.[2]
Spermidine is known to regulate plant growth, assisting the in vitro process of transcribing RNA, and inhibition of NOS. Also, spermidine is a precursor to other polyamines, such as spermine and thermospermine, some of which contribute to tolerance against drought and salinity in plants.
Spermidine has been tested and discovered to encourage hair shaft elongation and lengthen hair growth. Spermidine has also been found to “upregulate expression of the epithelial stem cell-associated keratins K15 and K19, and dose-dependently modulated K15 promoter activity in situ and the colony forming efficiency, proliferation and K15 expression of isolated human K15-GFP+ cells in vitro.”
Biochemical actions
Spermidine's known actions include:
- Inhibits neuronal nitric oxide synthase (nNOS)[3]
- Binds and precipitates DNA[4]
- Polyamine plant growth regulator[5][6][7][8][9][10][11]
Sources
Good dietary sources of spermidine are aged cheese, mushrooms, soy products, legumes, corn, and whole grains.[12]
| Food | Spermidine mg/kg | notes & refs |
|---|---|---|
| Chickpea | ~29 | [12] |
| Green peas | 46.6 | [12] |
| Soybean,dried | 128 | German [12] |
| Soybean,dried | 207 | Japanese [12] |
| chicken liver | 48.7 | [12] |
| Cheddar, 1yr old | 199.5 | [12] |
| Cauliflower (cooked) | ~25 | [12] |
| Broccoli (cooked) | ~25 | [12] |
| Mushroom | 88.6 | Japanese [12] |
| Mango | 30 | [12] |
| Rice bran | 50 | [12] |
Note - spermidine content varies by source and age. See ref for details.
In grains, the endosperm contains most of the spermidine. One of the best known grain dietary sources is wheat germ, containing as much as 243 mg/kg.[13]
Uses
- Spermidine can be used in electroporation while transferring the DNA into the cell under the electrical impulse. May be used for purification of DNA-binding proteins.
- Spermidine is also used, along with calcium chloride, for precipitating DNA onto microprojectiles for bombardment with a gene gun.[14]
- Spermidine has also been reported to protect the heart from aging and prolong the lifespan of mice, while in humans it was correlated with lower blood pressure.[15] It also was found to reduce the amount of aging in yeast, flies, worms, and human immune cells by inducing autophagy.[16] Recently Tirupathi Pichiah et al., suggested that spermidine may be helpful for treating type 2 diabetes.[17]
- Spermidine is commonly used for in vitro molecular biology reactions, particularly, in vitro transcription by Phage RNA polymerases, in vitro transcription by human RNA polymerase II (in vitro transcription by human RNA polymerase II, and in vitro translation.
- Spermidine increases specificity and reproducibility of Taq-mediated PCR by neutralizing and stabilizing the negative charge on DNA phosphate backbone.
- Spermidine is a polycationic reagent that aids in enzyme digestion by forcing apart DNA molecules.
See also
References
- ↑ American Heritage Dictionary Retrieved 2014-11-18.
- ↑ Minois, Nadège (28 January 2014). "Molecular Basis of the "Anti-Aging" Effect of Spermidine and Other Natural Polyamines – A Mini-Review". Gerontology. 60 (4): 319–326. doi:10.1159/000356748. PMID 24481223.
- ↑ Hu, J; Mahmoud, MI; El-Fakahany, EE (1994). "Polyamines inhibit nitric oxide synthase in rat cerebellum". Neuroscience Letters. 175 (1–2): 41–5. doi:10.1016/0304-3940(94)91073-1. PMID 7526294.
- ↑ Wan, CY; Wilkins, TA (1993). "Spermidine facilitates PCR amplification of target DNA". PCR methods and applications. 3 (3): 208–10. doi:10.1101/gr.3.3.208. PMID 8118404.
- ↑ Cull, M; McHenry, CS (1990). "Preparation of extracts from prokaryotes". Methods in enzymology. Methods in Enzymology. 182: 147–53. doi:10.1016/0076-6879(90)82014-S. ISBN 978-0-12-182083-1. PMID 2107372.
- ↑ Blethen, SL; Boeker, EA; Snell, EE (1968). "Argenine decarboxylase from Escherichia coli. I. Purification and specificity for substrates and coenzyme". The Journal of Biological Chemistry. 243 (8): 1671–7. PMID 4870599.
- ↑ Wu, WH; Morris, DR (1973). "Biosynthetic arginine decarboxylase from Escherichia coli. Subunit interactions and the role of magnesium ion". The Journal of Biological Chemistry. 248 (5): 1696–9. PMID 4571774.
- ↑ Tabor, CW; Tabor, H (1984). "Polyamines". Annual Review of Biochemistry. 53: 749–90. doi:10.1146/annurev.bi.53.070184.003533. PMID 6206782.
- ↑ Krug, MS; Berger, SL (1987). "First-strand cDNA synthesis primed with oligo(dT)". Methods in enzymology. Methods in Enzymology. 152: 316–25. doi:10.1016/0076-6879(87)52036-5. ISBN 978-0-12-182053-4. PMID 2443800.
- ↑ Karkas, JD; Margulies, L; Chargaff, E (1975). "A DNA polymerase from embryos of Drosophila melanogaster. Purification and properties". The Journal of Biological Chemistry. 250 (22): 8657–63. PMID 241752.
- ↑ Bouché, JP (1981). "The effect of spermidine on endonuclease inhibition by agarose contaminants". Analytical Biochemistry. 115 (1): 42–5. doi:10.1016/0003-2697(81)90519-4. PMID 6272602.
- 1 2 3 4 5 6 7 8 9 10 11 12 Ali, Mohamed Atiya; Poortvliet, Eric; Strömberg, Roger; Yngve, Agneta (2011). "Polyamines in foods: development of a food database". Food Nutr Res. 55: 5572. doi:10.3402/fnr.v55i0.5572. PMID 21249159.
- ↑ "Brochure on Polyamines, rev. 2" (PDF). Japan: Oryza Oil & Fat Chemocial Co., Ltd. 2011-12-26. Retrieved 2013-11-06.
- ↑ T.M. Klein; T. Gradziel; M.E. Fromm; J.C. Sanford (1988). "Factors influencing gene delivery into Zea mays cells by high–velocity microprojectiles,". Nature Biotechnology. 6 (5): 559–63. doi:10.1038/nbt0588-559.
- ↑ Eisenberg, Tobias; Abdellatif, Mahmoud; Schroeder, Sabrina; Primessnig, Uwe; Stekovic, Slaven; Pendl, Tobias; Harger, Alexandra; Schipke, Julia; Zimmermann, Andreas. "Cardioprotection and lifespan extension by the natural polyamine spermidine". Nature Medicine. doi:10.1038/nm.4222.
- ↑ Tobias Eisenberg, Heide Knauer, Alexandra Schauer, Sabrina Büttner, Christoph Ruckenstuhl, Didac Carmona-Gutierrez, Julia Ring, Sabrina Schroeder, Christoph Magnes, Lucia Antonacci, Heike Fussi, Luiza Deszcz, Regina Hart, Elisabeth Schraml, Alfredo Criollo, Evgenia Megalou, Daniela Weiskopf, Peter Laun, Gino Heeren, Michael Breitenbach, Beatrix Grubeck-Loebenstein, Eva Herker, Birthe Fahrenkrog, Kai-Uwe Fröhlich, Frank Sinner, Nektarios Tavernarakis, Nadege Minois, Guido Kroemer, Frank Madeo (4 October 2009). "Induction of autophagy by spermidine promotes longevity,". Nature Cell Biology. 11 (11): 1305–14. doi:10.1038/ncb1975. PMID 19801973.
- ↑ Tirupathi Pichiah, PB; Suriyakalaa, U; Kamalakkannan, S; Kokilavani, P; Kalaiselvi, S; SankarGanesh, D; Gowri, J; Archunan, G; Cha, YS; Achiraman, S (October 2011). "Spermidine may decrease ER stress in pancreatic beta cells and may reduce apoptosis via activating AMPK dependent autophagy pathway". Medical Hypotheses. 77 (4): 677–9. doi:10.1016/j.mehy.2011.07.014. PMID 21831529.