Voltammetry
Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied.[1][2]
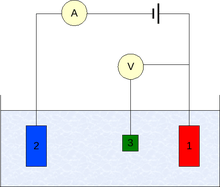
Three electrode system
Voltammetry experiments investigate the half-cell reactivity of an analyte. Voltammetry is the study of current as a function of applied potential. These curves I = f(E) are called voltammograms. The potential is varied arbitrarily either step by step or continuously, and the actual current value is measured as the dependent variable. The opposite, i.e., amperometry, is also possible but not common. The shape of the curves depends on the speed of potential variation (nature of driving force) and on whether the solution is stirred or quiescent (mass transfer). Most experiments control the potential (volts) of an electrode in contact with the analyte while measuring the resulting current (amperes).[3]
To conduct such an experiment one requires at least two electrodes. The working electrode, which makes contact with the analyte, must apply the desired potential in a controlled way and facilitate the transfer of charge to and from the analyte. A second electrode acts as the other half of the cell. This second electrode must have a known potential with which to gauge the potential of the working electrode, furthermore it must balance the charge added or removed by the working electrode. While this is a viable setup, it has a number of shortcomings. Most significantly, it is extremely difficult for an electrode to maintain a constant potential while passing current to counter redox events at the working electrode.
To solve this problem, the roles of supplying electrons and providing a reference potential are divided between two separate electrodes. The reference electrode is a half cell with a known reduction potential. Its only role is to act as reference in measuring and controlling the working electrode's potential and at no point does it pass any current. The auxiliary electrode passes all the current needed to balance the current observed at the working electrode. To achieve this current, the auxiliary will often swing to extreme potentials at the edges of the solvent window, where it oxidizes or reduces the solvent or supporting electrolyte. These electrodes, the working, reference, and auxiliary make up the modern three electrode system.
There are many systems which have more electrodes, but their design principles are generally the same as the three electrode system. For example, the rotating ring-disk electrode has two distinct and separate working electrodes, a disk and a ring, which can be used to scan or hold potentials independently of each other. Both of these electrodes are balanced by a single reference and auxiliary combination for an overall four electrode design. More complicated experiments may add working electrodes as required and at times reference or auxiliary electrodes.
In practice it can be important to have a working electrode with known dimensions and surface characteristics. As a result, it is common to clean and polish working electrodes regularly. The auxiliary electrode can be almost anything as long as it doesn't react with the bulk of the analyte solution and conducts well. It is (or was?) common to use mercury as working electrode e.g. DME and HMDE, and also as auxiliary, and the voltammetry method is then known as polarography. The reference is the most complex of the three electrodes; there are a variety of standards used and it is worth investigating elsewhere. For non-aqueous work, IUPAC recommends the use of the ferrocene/ferrocenium couple as an internal standard.[4] In most voltammetry experiments, a bulk electrolyte (also known as a supporting electrolyte) is used to minimize solution resistance. It is possible to run an experiment without a bulk electrolyte, but the added resistance greatly reduces the accuracy of the results. With room temperature ionic liquids, the solvent can act as the electrolyte.
Theory
Data analysis requires the consideration of kinetics in addition to thermodynamics, due to the temporal component of voltammetry. Idealized theoretical electrochemical thermodynamic relationships such as the Nernst equation are modeled without a time component. While these models are insufficient alone to describe the dynamic aspects of voltammetry, models like the Tafel equation and Butler-Volmer equation lay the groundwork for the modified voltammetry relationships that relate theory to observed results.[5]
Types of voltammetry
- Linear sweep voltammetry
- Staircase voltammetry
- Squarewave voltammetry
- Cyclic voltammetry - A voltammetric method that can be used to determine diffusion coefficients and half cell reduction potentials.
- Anodic stripping voltammetry - A quantitative, analytical method for trace analysis of metal cations. The analyte is deposited (electroplated) onto the working electrode during a deposition step, and then oxidized during the stripping step. The current is measured during the stripping step.
- Cathodic stripping voltammetry - A quantitative, analytical method for trace analysis of anions. A positive potential is applied, oxidizing the mercury electrode and forming insoluble precipitates of the anions. A negative potential then reduces (strips) the deposited film into solution.
- Adsorptive stripping voltammetry - A quantitative, analytical method for trace analysis. The analyte is deposited simply by adsorption on the electrode surface (i.e., no electrolysis), then electrolyzed to give the analytical signal. Chemically modified electrodes are often used.
- Alternating current voltammetry
- Polarography - a subclass of voltammetry where the working electrode is a dropping mercury electrode (DME), useful for its wide cathodic range and renewable surface.
- Rotated electrode voltammetry - A hydrodynamic technique in which the working electrode, usually a rotating disk electrode (RDE) or rotating ring-disk electrode (RRDE), is rotated at a very high rate. This technique is useful for studying the kinetics and electrochemical reaction mechanism for a half reaction.
- Normal pulse voltammetry
- Differential pulse voltammetry
- Chronoamperometry
History
The beginning of voltammetry was facilitated by the discovery of polarography in 1922 by the Nobel Prize–winning chemist Jaroslav Heyrovský. Early voltammetric techniques had many problems, limiting their viability for everyday use in analytical chemistry. In polarography, these problems included the fact that Mercury is oxidized at a potential that is more positive than +0.2, which makes it harder to analyze that results for the analytes in the positive region of the potential. Another problem included the residual current obtained from the charging of the large capacitance of the electrode surface.[6] When Heyrovsky first recorded the first dependence on the current flowing through the dropping mercury electrode on the applied potential in 1922, he took point-by-point measurements and he plotted a current-voltage curve. This was considered to be the first polarogram. In order to facilitate this process, he constructed what is now known as a polarograph with M. Shikata and, with enabled him to record photographically the same curve in a matter of hours. He gave recognition to the importance of potential and its control and also recognized the opportunities of measuring the limiting currents. He was also an important part of the introduction of dropping mercury electrode as a modern-day tool.[7]
In 1942 Archie Hickling built the first three electrodes potentiostat, which was an advancement for the field of electrochemistry.[8] He used this potentiostat to control the voltage of an electrode. In the meantime, the late 1940s, Kenneth Cole invented an electronic circuit which he called a voltage clamp. The voltage clamp was used to analyze the ionic conduction in nerves.
The 1960s and 1970s saw many advances in the theory, instrumentation, and the introduction of computer added and controlled systems. Modern polarographic and voltammetric methods on mercury electrodes came about in three sections.
The first section includes the development of the mercury electrodes. The following electrodes were produced: dropping mercury electrode, mercury steaming electrode, hanging mercury drop electrode, static mercury drop electrode, mercury film electrode, mercury amalgam electrodes, mercury microelectrodes, chemically modified mercury electrodes, controlled growth mercury electrodes, and contractible mercury drop electrodes.
There was also an advancement of the measuring techniques used. These measuring techniques include : classical DC polarography, oscillopolarography, Kaloussek’s switcher, AC polarography, tast polarography, normal pulse polarography, differential pulse polarography, square-wave voltammetry, cyclic voltammetry, anodic stripping voltammetry, convolution techniques, and elimination methods.
Lastly, there was also an advancement of preconcentration techniques that produced an increased the sensitivity of the mercury electrodes. This came about through the development of anodic stripping voltammetry, cathodic stripping voltammetry and adsorptive stripping voltammetry.[7]
These advancements improved sensitivity and created new analytical methods, which prompted the industry to respond with the production of cheaper potentiostat, electrodes, and cells that could be effectively used in routine analytical work.
Applications
Voltammetric sensors
A number of voltammetric systems are produced commercially for the determination of specific species that are of interest in industry and research. These devices are sometimes called electrodes but are, in fact, complete voltammetric cells and are better referred to as sensors. These sensors can be employed for the analysis of various organic and inorganic analytes in various matrices.[9]
The oxygen electrode
The determination of dissolved oxygen in a variety of aqueous environments, such as sea water, blood, sewage, effluents from chemical plants, and soils is of tremendous importance to industry, biomedical and environmental research, and clinical medicine. One of the most common and convenient methods for making such measurements is with the Clark oxygen sensor, which was patented by L.C. Clark, Jr. in 1956.
See also
References
- ↑ Kissinger, Peter; William R. Heineman (1996-01-23). Laboratory Techniques in Electroanalytical Chemistry, Second Edition, Revised and Expanded (2 ed.). CRC. ISBN 0-8247-9445-1.
- ↑ Zoski, Cynthia G. (2007-02-07). Handbook of Electrochemistry. Elsevier Science. ISBN 0-444-51958-0.
- ↑ Bard, Allen J.; Larry R. Faulkner (2000-12-18). Electrochemical Methods: Fundamentals and Applications (2 ed.). Wiley. ISBN 0-471-04372-9.
- ↑ Gritzner, G.; J. Kuta (1984). "Recommendations on reporting electrode potentials in nonaqueous solvents". Pure Appl. Chem. 56 (4): 461–466. doi:10.1351/pac198456040461. Retrieved 2009-04-17.
- ↑ Nicholson, R. S.; Irving. Shain (1964-04-01). "Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems.". Analytical Chemistry. 36 (4): 706–723. doi:10.1021/ac60210a007.
- ↑ Kounaves, Samuel. "Voltammetric Techniques". Tufts University Department of Chemistry
- 1 2 Barek, J. (2003). "Eighty Years of Polarography - History and Future". Electroanalysis. 15: 467–472.
- ↑ Hickling, A. (1942). "Studies in electrode polarisation. Part IV.-The automatic control of the potential of a working electrode". Transactions of the Faraday Society. 38: 27–33. doi:10.1039/TF9423800027.
- ↑ Sanghavi, Bankim; Srivastava, Ashwini (2010). "Simultaneous voltammetric determination of acetaminophen, aspirin and caffeine using an in situ surfactant-modified multiwalled carbon nanotube paste electrode". Electrochimica Acta. 55: 8638–8648. doi:10.1016/j.electacta.2010.07.093.
Further reading
- Reinmuth, W. H. (1961-11-01). "Theory of Stationary Electrode Polarography". Analytical Chemistry. 33 (12): 1793–1794. doi:10.1021/ac60180a004.
- Skoog, Douglas A.; Donald M. West; F. James Holler (1995-08-25). Fundamentals of Analytical Chemistry (7th ed.). Harcourt Brace College Publishers. ISBN 0-03-005938-0.
- Zanello, P. (2003-10-01). Inorganic Electrochemistry: Theory, Practice, and Application (1 ed.). Royal Society of Chemistry. ISBN 0-85404-661-5.
External links
- http://www.drhuang.com/science/chemistry/electrochemistry/polar.doc.htm
- http://new.ametek.com/content-manager/files/PAR/App%20Note%20E-4%20-%20Electrochemical%20Analysis%20Techniques1.pdf