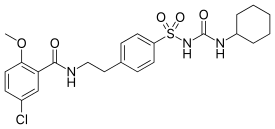
Glibenclamide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | see below |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a684058 |
| License data | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | A10BB01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Extensive |
| Metabolism | Hepatic hydroxylation (CYP2C9-mediated) |
| Biological half-life | 10 hours |
| Excretion | Renal and biliary |
| Identifiers | |
| |
| CAS Number |
10238-21-8 |
| PubChem (CID) | 3488 |
| IUPHAR/BPS | 2414 |
| DrugBank |
DB01016 |
| ChemSpider |
3368 |
| UNII |
SX6K58TVWC |
| KEGG |
D00336 |
| ChEBI |
CHEBI:5441 |
| ChEMBL |
CHEMBL472 |
| ECHA InfoCard | 100.030.505 |
| Chemical and physical data | |
| Formula | C23H28ClN3O5S |
| Molar mass | 494.004 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Glibenclamide (AAN, BAN, INN), also known as glyburide (USAN), is an antidiabetic drug in a class of medications known as sulfonylureas, closely related to sulfonamide antibiotics. It was developed in 1966 in a cooperative study between Boehringer Mannheim (now part of Roche) and Hoechst (now part of Sanofi-Aventis).[1]
Medical uses
It is used in the treatment of type 2 diabetes. As of 2003, in the United States, it was the most popular sulfonylurea.[2]
It is not as good as either metformin or insulin in those who have gestational diabetes.[3]
Side effects and contraindications
This drug is a major cause of drug-induced hypoglycemia. The risk is greater than with other sulfonylureas.[4] Cholestatic jaundice is noted.
Glibenclamide may be not recommended in those with G6PD deficiency, as it may cause acute haemolysis.[5]
Recently published data suggest glibenclamide is associated with significantly higher annual mortality when combined with metformin than other insulin-secreting medications, after correcting for other potentially confounding patient characteristics. The safety of this combination has been questioned.[6]
Mechanism of action
The drug works by binding to and inhibiting the ATP-sensitive potassium channels (KATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1) [7] in pancreatic beta cells. This inhibition causes cell membrane depolarization, opening voltage-dependent calcium channels. This results in an increase in intracellular calcium in the beta cell and subsequent stimulation of insulin release.
After a cerebral ischemic insult, the blood–brain barrier is broken and glibenclamide can reach the central nervous system. Glibenclamide has been shown to bind more efficiently to the ischemic hemisphere.[8] Moreover, under ischemic conditions SUR1, the regulatory subunit of the KATP- and the NCCa-ATP-channels, is expressed in neurons, astrocytes, oligodendrocytes, endothelial cells[9] and by reactive microglia.[8]
Analogs
Additional glibenclamide structural analogs have been prepared by Ahmadi et al.[10]
Research
Glibenclamide improves outcome in animal stroke models by preventing brain swelling[11] and enhancing neuroprotection.[7] A retrospective study showed, in type 2 diabetic patients already taking glyburide, NIH stroke scale scores were improved on discharge compared to diabetic patients not taking glyburide.[12]
Trade names
Glibenclamide is available as a generic, is manufactured by many pharmaceutical companies and is sold in doses of 1.25, 2.5 and 5 mg under many brand names including Gliben-J, Daonil, Diabeta, Euglucon, Gilemal, Glidanil, Glybovin, Glynase, Maninil, Micronase and Semi-Daonil. It is also available in a fixed-dose combination drug with metformin that is sold under various trade names, e.g. Bagomet Plus, Benimet, Glibomet, Gluconorm, Glucored, Glucovance, Metglib and many others.
See also
References
- ↑ Marble A (1971). "Glibenclamide, a new sulphonylurea: whither oral hypoglycaemic agents?". Drugs. 1 (2): 109–15. doi:10.2165/00003495-197101020-00001. PMID 4999930.
- ↑ Riddle MC (February 2003). "Editorial: sulfonylureas differ in effects on ischemic preconditioning--is it time to retire glyburide?". J. Clin. Endocrinol. Metab. 88 (2): 528–30. doi:10.1210/jc.2002-021971. PMID 12574174.
- ↑ Balsells, M; García-Patterson, A; Solà, I; Roqué, M; Gich, I; Corcoy, R (21 January 2015). "Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis.". BMJ (Clinical research ed.). 350: h102. doi:10.1136/bmj.h102. PMID 25609400.
- ↑ Gangji, A. S.; Cukierman, T.; Gerstein, H. C.; Goldsmith, C. H.; Clase, C. M. (1 February 2007). "A Systematic Review and Meta-Analysis of Hypoglycemia and Cardiovascular Events: A comparison of glyburide with other secretagogues and with insulin". Diabetes Care. 30 (2): 389–394. doi:10.2337/dc06-1789.
- ↑ Meloni G, Meloni T (January 1996). "Glyburide-induced acute haemolysis in a G6PD-deficient patient with NIDDM". Br. J. Haematol. 92 (1): 159–60. doi:10.1046/j.1365-2141.1996.275810.x. PMID 8562390.
- ↑ Monami M, Luzzi C, Lamanna C, Chiasserini V, Addante F, Desideri CM, Masotti G, Marchionni N, Mannucci E (2006). "Three-year mortality in diabetic patients treated with different combinations of insulin secretagogues and metformin". Diabetes Metab Res Rev. 22 (6): 477–82. doi:10.1002/dmrr.642. PMID 16634115.
- 1 2 Serrano-Martín X, Payares G, Mendoza-León A (December 2006). "Glibenclamide, a blocker of K+(ATP) channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis". Antimicrob. Agents Chemother. 50 (12): 4214–6. doi:10.1128/AAC.00617-06. PMC 1693980
 . PMID 17015627.
. PMID 17015627. - 1 2 Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, Carrasco JL, Batlle M, Pugliese M, Mahy N, Rodríguez MJ (May 2012). "ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats". Exp. Neurol. 235 (1): 282–96. doi:10.1016/j.expneurol.2012.02.010. PMID 22387180.
- ↑ Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V (September 2012). "Sulfonylurea receptor 1 in central nervous system injury: a focused review". J. Cereb. Blood Flow Metab. 32 (9): 1699–717. doi:10.1038/jcbfm.2012.91. PMC 3434627
 . PMID 22714048.
. PMID 22714048. - ↑ Ahmadi, A; Khalili, M; Khatami, K; Farsadrooh, M; Nahri-Niknafs, B (2014). "Synthesis and investigating hypoglycemic and hypolipidemic activities of some glibenclamide analogues in rats". Mini reviews in medicinal chemistry. 14 (2): 208–13. PMID 24387708.
- ↑ Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V (April 2006). "Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke". Nat. Med. 12 (4): 433–40. doi:10.1038/nm1390. PMC 2740734
 . PMID 16550187.
. PMID 16550187. - ↑ Kunte H, Schmidt S, Eliasziw M, del Zoppo GJ, Simard JM, Masuhr F, Weih M, Dirnagl U (September 2007). "Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke". Stroke. 38 (9): 2526–30. doi:10.1161/STROKEAHA.107.482216. PMC 2742413
 . PMID 17673715.
. PMID 17673715.