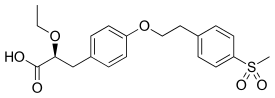
Tesaglitazar
 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
251565-85-2 |
| PubChem (CID) | 208901 |
| ChemSpider |
180999 |
| UNII |
6734037O3L |
| KEGG |
D01274 |
| ChEMBL |
CHEMBL521632 |
| ECHA InfoCard | 100.201.079 |
| Chemical and physical data | |
| Formula | C20H24O7S |
| Molar mass | 408.46 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Tesaglitazar (also known as AZ 242) is a dual peroxisome proliferator-activated receptor agonist with affinity to PPARα and PPARγ, proposed for the management of type 2 diabetes.[1]
The drug had completed several phase III clinical trials,[2] however in May, 2006 AstraZeneca announced that it had discontinued further development.[3]
References
- ↑ Wilding JP, Gause-Nilsson I, Persson A (2007). "Tesaglitazar, as add-on therapy to sulphonylurea, dose-dependently improves glucose and lipid abnormalities in patients with type 2 diabetes". Diab Vasc Dis Res. 4 (3): 194–203. doi:10.3132/dvdr.2007.040. PMID 17907109.
- ↑ "GALIDA (tesaglitazar) Clinical Trial Report Summaries". AstraZeneca. Retrieved 2008-03-17.
- ↑ "AstraZeneca Discontinues Development of GALIDA (tesaglitazar)". AstraZeneca. 2006-05-04. Retrieved 2012-07-23.
This article is issued from Wikipedia - version of the 11/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.