Sotalol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Betapace |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693010 |
| Pregnancy category | |
| Routes of administration | oral |
| ATC code | C07AA07 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90–100%[1] |
| Metabolism | Not metabolized[1] |
| Biological half-life | 12 hours[1] |
| Excretion |
Renal Lactic (In lactating females)[1] |
| Identifiers | |
| |
| CAS Number |
3930-20-9 |
| PubChem (CID) | 5253 |
| IUPHAR/BPS | 7297 |
| DrugBank |
DB00489 |
| ChemSpider |
5063 |
| UNII |
A6D97U294I |
| KEGG |
D08525 |
| ChEMBL |
CHEMBL471 |
| Chemical and physical data | |
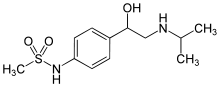
| Formula | C12H20N2O3S |
| Molar mass | 272.3624 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Sotalol is a drug used in individuals with rhythm disturbances of the heart (cardiac arrhythmias). It is a non-selective competitive beta-adrenergic receptor blocker that also exhibits Class III antiarrhythmic properties.[2][3] Originally discovered in 1960, sotalol became widely used first as a beta blocker in the 1980s, and its function as an antiarrhythmic drug was discovered soon after.[4] Due to the dual action of sotalol, it is often used preferentially to other beta blockers as treatment for both ventricular fibrillation and ventricular tachycardia.[2][5]
Trade names for Sotalol include Betapace and Betapace AF (Berlex Laboratories), Sotalex and Sotacor (Bristol-Myers Squibb), and Sotylize (Arbor Pharmaceuticals).[6] The U.S. Food and Drug Administration advises that sotalol only be used for serious arrhythmias, because its prolongation of the QT interval carries a small risk of life-threatening torsade de pointes.[6]
Medical uses
According to the U.S. Food and Drug Administration (FDA), sotalol can be validly used to maintain normal heart rhythm in people with life-threatening ventricular arrhythmias, or very symptomatic atrial fibrillation or flutter.[6] Due to the risk of serious side effects, the FDA states that sotalol should generally be reserved for patients whose ventricular arrhythmias are life-threatening, or whose fibrillation/flutter cannot be resolved using the Valsalva maneuver or another simple method.[6]
Mechanisms of action
Beta-blocker action
Sotalol non-selectively binds to both β1- and β2-adrenergic receptors preventing activation of the receptors by their stimulatory ligand.[2] Without the binding of this ligand to the receptor, the G-protein complex associated with the receptor cannot activate production of cyclic AMP, which is responsible for turning on calcium inflow channels.[7] A decrease in activation of calcium channels will therefore result in a decrease in intracellular calcium. In cardiac cells, calcium is important in generating electrical signals for contraction, as well as generating force for contraction.[8] In consideration of these important properties of calcium, two conclusions can be drawn. Firstly, with less calcium in the cell, there is a decrease in electrical signals for contraction, thus allowing time for the heart's natural pacemaker to rectify arrhythmic contractions.[4] Secondly, lower calcium means a decrease in strength and rate of the contractions, which can be helpful in treatment of abnormally high heart rates in patients with tachycardia.[4]
Type III antiarrhythmic action
Sotalol also acts on potassium channels and causes a delay in relaxation of the ventricles.[3] By blocking these potassium channels, sotalol inhibits efflux of K+ ions, which results in an increase in the time before another electrical signal can be generated in ventricular myocytes.[8] This increase in the period before a new signal for contraction is generated, helps to correct arrhythmias by reducing the potential for premature or abnormal contraction of the ventricles but also prolongs the frequency of ventricular contraction to help treat tachycardia.
Contraindications
According to the FDA, sotalol should not be used in people with a waking heart rate lower than 50 beats per minute.[6] It should not be used in people with sick sinus syndrome, long QT syndrome, cardiogenic shock, uncontrolled heart failure, bronchial asthma or a related bronchospastic condition, or people with serum potassium below 4 meq/L.[6] It should only be used in people with a second and third degree AV block if a functioning pacemaker is present.[6]
Since sotalol is removed from the body through the kidneys, it should not be used in people with a creatinine clearance rate below 40 mL/min.[6] It is also excreted in breast milk, so mothers should not breastfeed while taking sotalol.[6]
Because sotalol prolongs the QT interval, the FDA recommends against using it in conjunction with other drugs that prolong QT interval.[6] Studies have found serious side effects to be more common in patients also taking digoxin, possibly because of pre-existing heart failure in those patients.[6] As with other beta blockers, it may interact with calcium channel blockers, catecholamine-depleting drugs, insulin or antidiabetic drugs, beta2-adrenergic agonists, and Clonidine.[6]
Some evidence suggests that sotalol should be avoided in the setting of decreased ejection fraction due to an increased risk of death.[9]
Adverse effects
Over 10% of oral sotalol users experience fatigue, dizziness, lightheadedness, headache, weakness, nausea, shortness of breath, bradycardia (slow heart rate), palpitations, or chest pain. Risk for all of these effects increases with dosage.[1]
In rare cases, the QT prolongation caused by sotalol can lead to the development of life-threatening torsade de pointes (TdP) ventricular tachycardia. Across several trials, 0.6% of oral sotalol patients with supraventricular arrhythmias (such as atrial fibrillation) developed TdP.[1] For patients who had a history of sustained ventricular tachycardia (abnormal rhythm lasting more than 30 seconds), 4% developed TdP. Risk increases with dosage, female sex, or having a history of cardiomegaly or congestive heart failure.[1] The incidence of TdP for sustained ventricular tachycardia patients was 0% with an 80 mg daily dose, 0.5% at 160 mg, 1.6% at 320 mg, 4.4% at 480 mg, 3.7% at 640 mg, and 5.8% at doses greater than 640 mg.[1] Due to this risk, the U.S. Food and Drug Administration requires patients to be hospitalized for at least three days in a facility that can provide cardiac resuscitation and continuous electrocardiographic monitoring upon starting or restarting sotalol.[1]
History
Sotalol was first synthesized in 1960 by A. A. Larcen of Mead-Johnson Pharmaceutical.[10] It was originally recognized for its antihypertensive and antianginal effects.[11] It was made available in the United Kingdom and France in 1974, Germany in 1975, and Sweden in 1979.[11] In the 1980s, its antiarrhythmic properties were discovered.[11] The United States approved the drug in 1992.[12]
See also
References
- 1 2 3 4 5 6 7 8 9 U.S. Food and Drug Administration (July 2009). "Sotalol: Full Prescribing Information" (PDF). Retrieved 23 April 2015.
- 1 2 3 Bertrix, Lucien et al. Protection against ventricular and atrial fibrillation by sotalol. Cardiovascular Research 1986; 20, 358-363.
- 1 2 Edvardsson, N et al. Sotalol-induced delayed ventricular repolarization in man. European Heart Journal 1980; 1, 335-343
- 1 2 3 Antonaccio M, Gomoll A. Pharmacologic basis of the antiarrhythmic and hemodynamic effects of sotalol. Am J Cardiol 1993; 72, 27A-37A
- ↑ Dorian P, Newman D. Effect of sotalol on ventricular fibrillation and defibrillation in humans. Am J Cardiol 1993; 72, 72A-79A
- 1 2 3 4 5 6 7 8 9 10 11 12 "Sotylize: Full Prescribing Information" (PDF). Drugs@FDA. U.S. Food and Drug Administration. October 2014.
- ↑ Charnet P; et al. (1995). "cAMP-dependent phosphorylation of the cardiac L-type Ca channel: A missing link?". Biochimie. 77: 957–962. doi:10.1016/0300-9084(95)80008-5.
- 1 2 Kassotis, J et al. Beta receptor blockade potentiates the antiarryhthmic actions of d-sotalol on re-entrant ventricular tachycardia in a canine model of myocardial infarction. Journal of Cardiovascular Electrophysiology 2003; 14, 1233-1244
- ↑ Waldo A, Camm A, deRuyter H, Friedman P, MacNeil D, Pauls J, Pitt B, Pratt C, Schwartz P, Veltri E (1996). "Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol". Lancet. 348 (9019): 7–12. doi:10.1016/S0140-6736(96)02149-6. PMID 8691967.
- ↑ Hara, Takuji (2003). Innovation in the Pharmaceutical Industry: The Process of Drug Discovery and Development. Edward Elgar Publishing. p. 47.
- 1 2 3 Anderson JL, Askins JC, Gilbert EM, et al. (1986). "Multicenter trial of sotalol for suppression of frequent, complex ventricular arrhythmias: a double-blind, randomized, placebo-controlled evaluation of two doses". Journal of the American College of Cardiology. 8 (4): 752–762. doi:10.1016/S0735-1097(86)80414-4.
- ↑ Fernandes CM, Daya MR (1995). "Sotalol-induced bradycardia reversed by glucagon" (PDF). Canadian Family Physician. 41: 659–60, 663–5. PMC 2146520
 . PMID 7787496.
. PMID 7787496.