sec-Amyl acetate
Not to be confused with Amyl acetate.
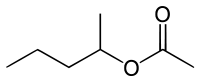 | |
| Names | |
|---|---|
| Preferred IUPAC name
Pentan-2-yl acetate | |
| Other names
1-Methylbutyl acetate 2-Pentanol acetate 2-Pentyl ester of acetic acid | |
| Identifiers | |
| 626-38-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 11775 |
| ECHA InfoCard | 100.009.952 |
| PubChem | 12278 |
| |
| |
| Properties | |
| C7H14O2 | |
| Molar mass | 130.19 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Odor | Mild,[1] like bananas[2] |
| Density | 0.87 g/mL (20°C)[1] |
| Melting point | −78 °C; −109 °F; 195 K [1] |
| Boiling point | 121 °C; 249 °F; 394 K [1] |
| 0.2g/100g water (20°C)[2] | |
| Vapor pressure | 7 mmHg (20°C)[1] |
| Hazards | |
| GHS signal word | Wng[2] |
| H226[2] | |
| Flash point | 32 °C; 89 °F; 305 K [1] |
| 380 | |
| Explosive limits | 1%-7.5% (20°C)[1] |
| Lethal dose or concentration (LD, LC): | |
| LCLo (lowest published) |
9200 ppm (guinea pig, 7 hr) 10,000 ppm (guinea pig, 5 hr)[3] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 125 ppm (650 mg/m3)[1] |
| REL (Recommended) |
TWA 125 ppm (650 mg/m3)[1] |
| IDLH (Immediate danger) |
1000 ppm[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
sec-Amyl acetate is an organic compound and an ester. It is formed in an esterification reaction of sec-amyl alcohol (2-pentanol) and acetic acid.[2] It reacts with nitrates, strong oxidizers, alkalis, and acids.[1]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "NIOSH Pocket Guide to Chemical Hazards #0032". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 5 "2-Pentyl Acetate". PubChem. NCBI.
- ↑ "sec-Amyl acetate". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
This article is issued from Wikipedia - version of the 11/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.