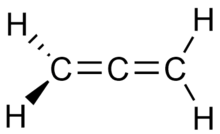
Propadiene
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Allene | |||
| Systematic IUPAC name
Propa-1,2-diene (substitutive) Dimethylenecarbon, dimethylenemethane (additive) | |||
| Identifiers | |||
| 463-49-0 | |||
| 3D model (Jmol) | Interactive image Interactive image | ||
| 1730774 | |||
| ChEBI | CHEBI:37601 | ||
| ChEMBL | ChEMBL116960 | ||
| ChemSpider | 9642 | ||
| ECHA InfoCard | 100.006.670 | ||
| EC Number | 207-335-3 | ||
| 860 | |||
| MeSH | Propadiene | ||
| PubChem | 10037 | ||
| UN number | 2200 | ||
| |||
| |||
| Properties | |||
| C3H4 | |||
| Molar mass | 40.07 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Melting point | −136 °C (−213 °F; 137 K) | ||
| Boiling point | −34 °C (−29 °F; 239 K) | ||
| log P | 1.45 | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| EU classification (DSD) |
| ||
| R-phrases | R12 | ||
| S-phrases | S9, S16, S33 | ||
| NFPA 704 | |||
| Explosive limits | 13% | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Propadiene is the organic compound with the formula H2C=C=CH2. It is the simplest allene or compound with two adjacent C=C double bonds, and can also be identified as allene.[1] As a constituent of MAPP gas, it has been used as a fuel for specialized welding.
Production and equilibrium with methylacetylene
Allene exists in equilibrium with methylacetylene (or propyne), the mixture sometimes being called MAPD for methylacetylene-propadiene:
- H3CC≡CH ⇌ H2C=C=CH2
for which Keq = 0.22 at 270 °C or 0.1 at 5 °C. MAPD is produced as a side product, often an undesirable one, of cracking propane to produce propene, an important feedstock in the chemical industry. MAPD interferes with the catalytic polymerization of propene.[2]
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "allenes".
- ↑ Klaus Buckl, Andreas Meiswinkel "Propyne" in Ullmann's Encyclopedia of Industrial Chemistry 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.m22_m01
This article is issued from Wikipedia - version of the 9/23/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.