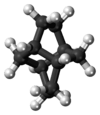
Twistane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
tricyclo[4.4.0.03,8]decane | |||
| Identifiers | |||
| 253-14-5 37165-28-8 (−) 21449-14-9 (+) | |||
| 3D model (Jmol) | Interactive image | ||
| |||
| |||
| Properties | |||
| C10H16 | |||
| Molar mass | 136.24 g·mol−1 | ||
| Melting point | 163 to 164.8 °C (325.4 to 328.6 °F; 436.1 to 437.9 K)[1] | ||
| Structure | |||
| D2 | |||
| 0 D | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Twistane (IUPAC name: tricyclo[4.4.0.03,8]decane[2]) is an organic compound with the formula C10H16.[3] It is a cycloalkane and an isomer of the simplest diamondoid, adamantane, and like adamantane, is not very volatile. Twistane was named for the way its rings are permanently forced into the cyclohexane conformation known as the "twist-boat".[1] The compound was first reported by Whitlock in 1962.[4]
Synthesis
Twistane has been synthesized in a variety of ways. The original 1962 method was based on a bicyclo[2.2.2]octane framework.[4] A 1967 publication concerned a intramolecular aldol condensation of a cis-decalin diketone.[5] It is formed when basketane is hydrogenated.[6]
Symmetry
The only symmetry operation in twistane is rotation, and there exists three 2-fold axes as shown in the left picture. Thus the point group of twistane is D2. Although twistane has four stereocenters, it only exists as two enantiomers. This is because it is symmetric along its C2 axis.[7]
Polytwistane
Polytwistane is a hypothetical polymer of fused twistane units awaiting actual synthesis.[8][9][10][11]
References
- 1 2 Beyer, Hans; Walter, Wolfgang; trans. Douglas Lloyd (1997), Organic Chemistry, Horwood Publishing, p. 416, ISBN 1-898563-37-3, retrieved 2008-12-09
- ↑ Quinkert, Gerhard; Egert, Ernst; Griesinger, Christian; trans. Andrew Beard (1996), Aspects of Organic Chemistry: Structure, Basel, Switzerland: Helvetica Chimica Acta, p. 107, ISBN 3-906390-15-2, retrieved 2008-12-09
- ↑ Classics in Hydrocarbon Chemistry: Syntheses, Concepts, Perspectives Henning Hopf ISBN 978-3-527-29606-4. 2000
- 1 2 Tricyclo[4.4.0.03.8]Decane H. W. Whitlock Journal of the American Chemical Society 1962 84 (17), 3412-3413 doi:10.1021/ja00876a047
- ↑ A new synthesis of twistane Jean Gauthier, Pierre Deslongchamps Canadian Journal of Chemistry, 1967, 45(3): 297-300, doi:10.1139/v67-052
- ↑ Ho, Tse-Lok (1995), Symmetry: A Basis for Synthesis Design, Wiley-IEEE, p. 69, ISBN 0-471-57376-0, retrieved 2008-12-10
- ↑ Kalsi, P. S. (2005), Stereochemistry Conformation and Mechanism, New Age Publishers, p. 94, ISBN 81-224-1564-4, retrieved 2008-12-10
- ↑ Barua, S. R., Quanz, H., Olbrich, M., Schreiner, P. R., Trauner, D. and Allen, W. D. (2014), Polytwistane. Chem. Eur. J., 20: 1638–1645. doi:10.1002/chem.201303081
- ↑ A step toward polytwistane: synthesis and characterization of C2-symmetric tritwistane Martin Olbrich, Peter Mayer and Dirk Trauner Org. Biomol. Chem., 2014,12, 108-112 doi:10.1039/C3OB42152J
- ↑ Synthetic Studies toward Polytwistane Hydrocarbon Nanorods Martin Olbrich, Peter Mayer, and Dirk Trauner The Journal of Organic Chemistry Article ASAP 2014 doi:10.1021/jo502618g
- ↑ Calculated Nuclear Magnetic Resonance Spectra of Polytwistane and Related Hydrocarbon Nanorods Boris Maryasin, Martin Olbrich, Dirk Trauner and Christian Ochsenfeld J. Chem. Theory Comput., 2015,11(3), 1020-1026 doi:10.1021/ct5011505