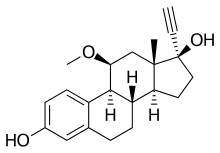
Moxestrol
 | |
| Clinical data | |
|---|---|
| Trade names | Surestryl |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | G03CB04 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 33%[1] |
| Protein binding | Minimal[1] |
| Biological half-life | 8.2 hours[1] |
| Identifiers | |
| |
| Synonyms | R-2858, RU-2858, NSC-118191 |
| CAS Number | 34816-55-2 |
| PubChem (CID) | 11954041 |
| ChemSpider | 10128336 |
| Chemical and physical data | |
| Formula | C21H26O3 |
| Molar mass | 326.42934 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Moxestrol (INN) (brand name Surestryl), also known as 11β-methoxy-17α-ethinyl-1,3,5(10)-estratriene-3,17β-diol or as 11β-methoxy-17α-ethinylestradiol, is a synthetic, steroidal estrogen that is or was used in Europe for the treatment of menopausal symptoms.[2][3][4][5] It is the 11β-methoxy derivative of ethinyl estradiol, and is one of the most potent estrogens known, being some 10–100 times more potent than estradiol and about 5-fold more potent than ethinyl estradiol.[4][5] The very high potency of moxestrol has been attributed to its high affinity for the estrogen receptor (ER), its negligible plasma binding to sex hormone binding globulin and low binding to serum albumin,[1] and its lower relative rate of metabolization.[4][5] In contrast to estradiol, which has roughly the same affinity for both ERs (Ki = 0.12 nM and 0.15 nM, respectively), moxestrol possesses several-fold selectivity for ERα (Ki = 0.50 nM) over ERβ (Ki = 2.6 nM).[6]
See also
References
- 1 2 3 4 Salmon, J.; Coussediere, D.; Cousty, C.; Raynaud, J.P. (1983). "Pharmacokinetics and metabolism of moxestrol in animals—rat, dog and monkey". Journal of Steroid Biochemistry. 19 (2): 1223–1234. doi:10.1016/0022-4731(83)90421-1. ISSN 0022-4731.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 841–. ISBN 978-1-4757-2085-3.
- ↑ Dr. Ian Morton; I.K. Morton; Judith M. Hall (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 186–. ISBN 978-0-7514-0499-9.
- 1 2 3 Jonathan J. Li; Satyabrata Nandi; Sara A. Li (6 December 2012). Hormonal Carcinogenesis: Proceedings of the First International Symposium. Springer Science & Business Media. pp. 184–. ISBN 978-1-4613-9208-8.
- 1 2 3 Adrain D. Nunn (19 June 1992). Radiopharmaceuticals: Chemistry and Pharmacology. CRC Press. pp. 342–. ISBN 978-0-8247-8624-3.
- ↑ Lund TD, Hinds LR, Handa RJ (2006). "The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus". J. Neurosci. 26 (5): 1448–56. doi:10.1523/JNEUROSCI.3777-05.2006. PMID 16452668.