Flavonols
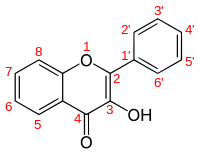
Flavonols are a class of flavonoids that have the 3-hydroxyflavone backbone (IUPAC name : 3-hydroxy-2-phenylchromen-4-one). Their diversity stems from the different positions the phenolic -OH groups. They are distinct from flavanols (with "a") such as catechin, another class of flavonoids.
Flavonols are present in a wide variety of fruits and vegetables. In Western populations, estimated daily intake is in the range of 20–50 mg per day for flavonols. Individual intake varies depending on the type of diet consumed.[1]
The phenomenon of dual fluorescence (due to excited state intramolecular proton transfer or ESIPT) is induced by tautomerism of flavonols (and glucosides) and could contribute to plant UV protection and flower colour.[2]
Besides being a subclass of flavonoids (health effects), flavonols are suggested by a study of cranberry juice to play a role along with proanthocyanidins, in the juice's ability to block bacterial adhesion, demonstrated by the compressing the fimbria of E. coli bacteria in the urinary tract so as to greatly reduce the ability of those bacteria to stay put and initiate an infection.[3] Flavonol aglycones in plants are potent antioxidants that serve to protect the plant from reactive oxygen species (ROS).[4]
Flavonols
| Name | IUPAC name | 5 | 6 | 7 | 8 | 2' | 3' | 4' | 5' | 6' |
|---|---|---|---|---|---|---|---|---|---|---|
| 3-Hydroxyflavone | 3-hydroxy-2-phenylchromen-4-one | H | H | H | H | H | H | H | H | H |
| Azaleatin | 2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-5-methoxychromen-4-one | OCH3 | H | OH | H | H | H | OH | OH | H |
| Fisetin | 3,3',4',7-tetrahydroxy-2-phenylchromen-4-one | H | H | OH | H | H | H | OH | OH | H |
| Galangin | 3,5,7-trihydroxy-2-phenylchromen-4-one | OH | H | OH | H | H | H | H | H | H |
| Gossypetin | 2-(3,4-dihydroxyphenyl)-3,5,7,8-tetrahydroxychromen-4-one | OH | H | OH | OH | H | OH | OH | H | H |
| Kaempferide | 3,5,7-trihydroxy-2-(4-methoxyphenyl)chromen-4-one | OH | H | OH | H | H | H | OCH3 | H | H |
| Kaempferol | 3,4',5,7-tetrahydroxy-2-phenylchromen-4-one | OH | H | OH | H | H | H | OH | H | H |
| Isorhamnetin | 3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one | OH | H | OH | H | H | OCH3 | OH | H | H |
| Morin | 2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one | OH | H | OH | H | OH | H | OH | H | H |
| Myricetin | 3,3',4',5',5,7-hexahydroxy-2-phenylchromen-4-one | OH | H | OH | H | H | OH | OH | OH | H |
| Natsudaidain | 2-(3,4-dimethoxyphenyl)-3-hydroxy-5,6,7,8-tetramethoxychromen-4-one | OCH3 | OCH3 | OCH3 | OCH3 | H | H | OCH3 | OCH3 | H |
| Pachypodol | 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7-dimethoxychromen-4-one | OH | H | OCH3 | H | H | OCH3 | OH | H | H |
| Quercetin | 3,3',4',5,7-pentahydroxy-2-phenylchromen-4-one | OH | H | OH | H | H | OH | OH | H | H |
n
I
| Rhamnazin | 3,5-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-7-methoxychromen-4-one | OH | H | OCH3 | H | H | OCH3 | OH | H | H |
| Rhamnetin | 2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-7-methoxychromen-4-one | OH | H | OCH3 | H | H | OH | OH | H | H |
Flavonol glycosides
| Name | Aglycone | 3 | 5 | 6 | 7 | 8 | 2' | 3' | 4' | 5' | 6' |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Astragalin | Kaempferol | Glc | |||||||||
| Azalein | Azaleatin | Rha | |||||||||
| Hyperoside | Quercetin | Gal | |||||||||
| Isoquercitin | Quercetin | Glc | |||||||||
| Kaempferitrin | Kaempferol | Rha | Rha | ||||||||
| Myricitrin | Myricetin | Rha | |||||||||
| Quercitrin | Quercetin | Rha | |||||||||
| Robinin | Kaempferol | Robinose | Rha | ||||||||
| Rutin | Quercetin | Rutinose | |||||||||
| Spiraeoside | Quercetin | Glc | |||||||||
| Xanthorhamnin | Rhamnetin | trisaccharide | |||||||||
| Amurensin | Kaempferol | Glc | tert-amyl | ||||||||
| Icariin | Kaempferide | Rha | Glc | tert-amyl | |||||||
| Troxerutin | Quercetin | Rutinose | hydroxyethyl | hydroxyethyl | hydroxyethyl |
Drug interactions
Flavonoids have effects on CYP (P450) activity. Flavonols are inhibitor of CYP2C9 [5] and CYP3A4,[1] which are enzymes that metabolize most drugs in the body.
See also
References
- 1 2 Cermak R, Wolffram S (October 2006). "The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms". Curr. Drug Metab. 7 (7): 729–44. doi:10.2174/138920006778520570. PMID 17073577.
- ↑ Tautomerism of flavonol glucosides: relevance to plant UV protection and flower colour, Gerald J. Smith and Kenneth R. Markham. Journal of Photochemistry and Photobiology A: Chemistry, Volume 118, Issue 2, 30 October 1998, Pages 99-105
- ↑ http://medicalxpress.com/news/2016-07-juicy-news-cranberries.html
- ↑ Nakabayashi R1, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K. "Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids.".
- ↑ Si D, Wang Y, Zhou YH, et al. (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols". Drug Metab. Dispos. 37 (3): 629–34. doi:10.1124/dmd.108.023416. PMID 19074529.