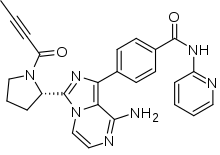
Acalabrutinib
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 1420477-60-6 |
| ChemSpider | 36764951 |
| Chemical and physical data | |
| Formula | C26H23N7O2 |
| Molar mass | 465.507 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Acalabrutinib (rINN,[1] ACP-196) is a novel experimental anti-cancer drug and a 2nd generation Bruton's tyrosine kinase (BTK) inhibitor[2][3] developed by Acerta Pharma.[4] It is more potent and selective (fewer side-effects) than ibrutinib, the first-in-class BTK inhibitor.[2][3][5]
Clinical and Regulatory Status
Pre-clinical
Relative to ibrutinib, acalabrutinib demonstrated higher selectivity and inhibition of the targeted activity of BTK, while having a much greater IC50 or otherwise virtually no inhibition on the kinase activities of ITK, EGFR, ERBB2, ERBB4, JAK3, BLK, FGR, FYN, HCK, LCK, LYN, SRC, and YES1.[3] In addition, in platelets treated with ibrutinib, thrombus formation was clearly inhibited while no impact to thrombus formation was identified relative to controls for those treated with acalabrutinib.[3] These findings strongly suggest an improved safety profile of acalabrutinib with minimized adverse effects relative to ibrutinib.[3]
As was conducted in the development of ibrutinib, pre-clinical studies of acalabrutinib included in vitro and in vivo pharmacodynamic evaluation in a canine lymphoma model.[6] A dose-dependent relationship resulting in cyto-toxicity and anti-proliferative effects was first demonstrated in a canine lymphoma cell line in vitro.[6] In vivo, the compound was found to be generally safe and well tolerated in the dosage range of 2.5–20 mg/kg every 12 or 24 hours, with clinical benefit observed in 30% of canine patients while observed adverse events consisted primarily of gastrointestinal effects such as anorexia, weight loss, vomiting, diarrhea and lethargy.[6]
Clinical
The interim results of the still on-going first human phase 1/2 clinical trial (NCT02029443) with 61 patients for the treatment of relapsed chronic lymphocytic leukemia (CLL) are encouraging, with a 95% overall response rate demonstrating potential to become a best-in-class treatment for CLL.[2][7] Notably, a 100% response rate was achieved for those patients which were positive for the 17p13.1 gene deletion - a subgroup of patients that typically results in a poor response to therapy and expected outcomes.[3]
The most common adverse events were headache, diarrhea and weight gain.[3] Despite the appearance of a greater occurrence of transient headaches, the pre-clinical data suggests a preferred advantage of acalabrutinib over ibrutinib due to expected reduced adverse events of skin rash, severe diarrhea, and bleeding risk.[3] An additional clinical trial is currently in progress to directly compare the safety and efficacy performance of acalabrutinib to ibrutinib to better elucidate the differences in the therapeutic agents.[3]
While the primary indication is for CLL, as of late 2016, acalabrutinib is under evaluation for multiple indications in 20+ clinical trials (alone and in combination with other interventions) for various blood cancers, solid tumors, and rheumatoid arthritis.[7][8] Approximately 1,000 patients have been treated with acalabrutinib in clinical trials so far, including more than 600 on acalabrutinib alone and almost 400 on additional therapies in combination with acalabrutinib.[9]
Regulatory
As of February 2016, acalabrutinib had received orphan designation in the United States for CLL only,[10] and was similarly designated as an orphan medicinal product by the European Medicines Agency (EMA) Committe for Orphan Medicinal Products (COMP) for treatment of three indications - chronic lymphocytic leukemia (CLL)/ small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), and lymphoplasmacytic lymphoma (Waldenström's macroglobulinaemia, MG).[11] If the drug is ultimately approved, this designation will result in a 10-year period of market exclusivity for the stated indications within Europe.[12]
Commercial Aspects
Acerta Pharma, the innovator responsible for the discovery and development of acalabrutinib, is a clinical stage biopharmaceutical company recently founded in 2012 in Oss, the Netherlands.[13] A combined $13 Million in Series A funding was secured March 14, 2013 from various investor sources including the venture capital firms of BioGeneration Ventures and OrbiMed Advisors, the Dutch State and Province of Brabant through the Brabant Development Agency, and the private US equity firm Frazier Healthcare.[14] Further undisclosed amounts of Series B funding was secured May 2015 from the mutual fund company T. Rowe Price.[15]
After the promising results for the treatment of CLL in initial clinical trials,[2] Astra Zeneca purchased a 55% stake in Acerta Pharma for $4 billion in December 2015, with an option to acquire the remaining 45% stake for an additional $3 billion, conditional on the first approval in both the US and Europe and the establishment of commercial opportunity.[16]
Intellectual Property
Several patents have been filed by Acerta Pharma through the World Intellectual Property Organization (WIPO) for the use of acalabrutinib (and structurally similar derivatives) either alone or in combination with additional therapeutic agents for the treatment of various hematological and solid tumor cancers as well as inflammatory and autoimmune diseases.[17][18][19][19][20][21][22][23][24][25][26][27]
Notably, patents filed through WIPO still need to be filed appropriately for each individual nation on the path to commercialization. For example, one related United States patent application is US2014155385, which was filed July 11, 2012 and approved June 5th, 2014 for the use of 6-5 membered fused pyridine ring compounds (including acalabrutnib and its structurally similar derivatives) in the treatment of BTK mediated disorders.[28]
References
- ↑ "WHO Drug Information - recommended INN" (PDF). WHO Drug Information. World Health Oorganisation. Retrieved 24 December 2015.
- 1 2 3 4 Byrd; et al. (2015). "Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia.". doi:10.1056/NEJMoa1509981.
- 1 2 3 4 5 6 7 8 9 Wu, Jingjing; Zhang, Mingzhi; Liu, Delong (2016-01-01). "Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor". Journal of Hematology & Oncology. 9: 21. doi:10.1186/s13045-016-0250-9. ISSN 1756-8722. PMC 4784459
 . PMID 26957112.
. PMID 26957112. - ↑ "AstraZeneca to buy Acerta for blood cancer drug". www.rsc.org. Chemistry World - Royal Society of Chemistry. Retrieved 24 December 2015.
- ↑ Wu, Jingjing; Zhang, Mingzhi; Liu, Delong (2016-03-09). "Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor". Journal of Hematology & Oncology. 9 (1). doi:10.1186/s13045-016-0250-9. ISSN 1756-8722. PMC 4784459
 . PMID 26957112.
. PMID 26957112. - 1 2 3 Harrington, Bonnie K.; Gardner, Heather L.; Izumi, Raquel; Hamdy, Ahmed; Rothbaum, Wayne; Coombes, Kevin R.; Covey, Todd; Kaptein, Allard; Gulrajani, Michael (2016-07-19). "Preclinical Evaluation of the Novel BTK Inhibitor Acalabrutinib in Canine Models of B-Cell Non-Hodgkin Lymphoma". PLOS ONE. 11 (7): e0159607. doi:10.1371/journal.pone.0159607. ISSN 1932-6203. PMC 4951150
 . PMID 27434128.
. PMID 27434128. - 1 2 Acerta Pharma Announces Study Published in New England Journal of Medicine Demonstrates Acalabrutinib (ACP-196) Shows Marked Activity in Relapsed Chronic Lymphocytic Leukemia
- ↑ 21 studies found for: ACP-196
- ↑ "Acerta Investor Conference Call - 17 December 2015" (PDF). www.astrazeneca.com. Retrieved 2016-11-20.
- ↑ "Public summary of opinion on orphan designation" (PDF). European Medicines Agency. 2016-04-27. Retrieved 2016-11-20.
- ↑ "azn201602256k.htm". www.sec.gov. Retrieved 2016-11-21.
- ↑ House, SA Editor Douglas W. (2016-02-25). "AstraZeneca and Acerta Pharma's acalabrutinib tagged an Orphan Drug in Europe for three indications". Seeking Alpha. Retrieved 2016-11-21.
- ↑ "Acerta Pharma B.V. - Company Profile - BioCentury". www.biocentury.com. Retrieved 2016-11-12.
- ↑ "Log in to CB Insights". www.cbinsights.com. Retrieved 2016-11-12.
- ↑ "This is The Most Valuable Startup You've Never Heard Of". Fortune. 2015-12-17. Retrieved 2016-11-12.
- ↑ Walker, Ian; Roland, Denise (2015-12-17). "AstraZeneca to Buy Stake in Acerta Pharma". Wall Street Journal. ISSN 0099-9660. Retrieved 2016-11-19.
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Nov 26, 2015), Btk inhibitor for the treatment of chronic lymphocytic and small lymphocytic leukemia, retrieved 2016-11-19
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Jun 11, 2015), Therapeutic combination of a pi3k inhibitor and a btk inhibitor, retrieved 2016-11-19
- 1 2 IZUMI, Raquel; SALVA, Francisco; HAMDY, Ahmed (Feb 4, 2016), Methods of blocking the cxcr-4/sdf-1 signaling pathway with inhibitors of bruton's tyrosine kinase, retrieved 2016-11-19
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Aug 4, 2016), Therapeutic combinations of a btk inhibitor, a pi3k inhibitor and/or a jak-2 inhibitor, retrieved 2016-11-19
- ↑ Lannutti, Brian; Covey, Todd; Kaptein, Allard; Johnson, David; STAMATIS, Jay; Krejsa, Cecile M.; Slatter, John Gregory (Feb 11, 2016), Methods of treating cancers, immune and autoimmune diseases, and inflammatory diseases based on btk occupancy and btk resynthesis rate, retrieved 2016-11-19
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Feb 18, 2016), Btk inhibitors to treat solid tumors through modulation of the tumor microenvironment, retrieved 2016-11-19
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Feb 18, 2016), Therapeutic combinations of a btk inhibitor, a pi3k inhibitor, a jak-2 inhibitor, and/or a bcl-2 inhibitor, retrieved 2016-11-19
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Feb 18, 2016), Therapeutic combinations of a btk inhibitor, a pi3k inhibitor, a jak-2 inhibitor, a pd-1 inhibitor and/or a pd-l1 inhibitor, retrieved 2016-11-19
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Feb 18, 2016), Therapeutic combinations of a btk inhibitor, a pi3k inhibitor, a jak-2 inhibitor and/or a cdk 4/6 inhibitor, retrieved 2016-11-19
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Jul 28, 2016), Compositions and methods for treatment of chronic lymphocytic leukemia and small lymphocytic leukemia using a btk inhibitor, retrieved 2016-11-19
- ↑ HAMDY, Ahmed; Rothbaum, Wayne; IZUMI, Raquel; Lannutti, Brian; Covey, Todd; ULRICH, Roger; Johnson, Dave; Barf, Tjeerd; Kaptein, Allard (Aug 18, 2016), Therapeutic combinations of a btk inhibitor, a pi3k inhibitor, a jak-2 inhibitor, a pd-1 inhibitor, and/or a pd-l1 inhibitor, retrieved 2016-11-19
- ↑ Barf, Tjeerd A.; Jans, Christian Gerardus Johannes Maria; Man, Petrus Antonius De Adrianus; Oubrie, Arthur A.; Raaijmakers, Hans C. A.; Rewinkel, Johannes Bernardus Maria; Sterrenburg, Jan-Gerard; Wijkmans, Jacobus C. H. M. (5 June 2014), United States Patent Application: 0140155385 - 4-IMIDAZOPYRIDAZIN-1-YL-BENZAMIDES AND 4-IMIDAZOTRIAZIN-1-YL-BENZAMIDES AS BTK INHIBITORS, retrieved 2016-11-19