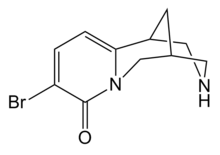
3-Bromocytisine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 207390-14-5 |
| PubChem (CID) | 15519735 |
| ChemSpider | 21376088 |
| Chemical and physical data | |
| Formula | C11H13BrN2O |
| Molar mass | 269.14 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
3-Bromocytisine is a derivative of the toxic alkaloid cytisine that acts as a highly potent agonist at neural nicotinic acetylcholine receptors, binding primarily to the α4β2 and α7 subtypes. 3-Bromocytisine is a full agonist at the α7 subtype while it is only a partial agonist at α4β2, but has an extremely strong binding affinity at α4β2 with 200-fold selectivity for α4β2 over α7. In animal studies 3-bromocytisine stimulates the release of dopamine and noradrenaline and increases locomotor activity.[1][2][3][4]
References
- ↑ Imming, P.; Klaperski, P.; Stubbs, M. T.; Seitz, G.; Gündisch, D. (2001). "Syntheses and evaluation of halogenated cytisine derivatives and of bioisosteric thiocytisine as potent and selective nAChR ligands". European journal of medicinal chemistry. 36 (4): 375–388. doi:10.1016/S0223-5234(01)01222-3. PMID 11461763.
- ↑ Houlihan, L. M.; Slater, Y.; Guerra, D. L.; Peng, J. H.; Kuo, Y. P.; Lukas, R. J.; Cassels, B. K.; Bermudez, I. (2001). "Activity of cytisine and its brominated isosteres on recombinant human alpha7, alpha4beta2 and alpha4beta4 nicotinic acetylcholine receptors". Journal of Neurochemistry. 78 (5): 1029–1043. doi:10.1046/j.1471-4159.2001.00481.x. PMID 11553677.
- ↑ Abin-Carriquiry, J. A. S.; Voutilainen, M. H.; Barik, J.; Cassels, B. K.; Iturriaga-Vásquez, P.; Bermudez, I.; Durand, C.; Dajas, F.; Wonnacott, S. (2006). "C3-halogenation of cytisine generates potent and efficacious nicotinic receptor agonists". European Journal of Pharmacology. 536 (1–2): 1–11. doi:10.1016/j.ejphar.2006.02.012. PMID 16563372.
- ↑ Abin-Carriquiry, J. A. S.; Urbanavicius, J.; Scorza, C.; Rebolledo-Fuentes, M.; Wonnacott, S.; Cassels, B. K.; Dajas, F. (2010). "Increase in locomotor activity after acute administration of the nicotinic receptor agonist 3-bromocytisine in rats". European Journal of Pharmacology. 634 (1–3): 89–94. doi:10.1016/j.ejphar.2010.02.030. PMID 20184877.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.