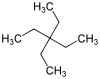
Tetraethylmethane
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
3,3-Diethylpentane[1] | |||
| Identifiers | |||
| 1067-20-5 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 13402 | ||
| ECHA InfoCard | 100.151.290 | ||
| PubChem | 14020 | ||
| |||
| |||
| Properties | |||
| C9H20 | |||
| Molar mass | 128.26 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Odourless | ||
| Density | 724 mg mL−1 | ||
| Melting point | −34 to −30 °C; −29 to −22 °F; 239 to 243 K | ||
| Boiling point | 145.8 to 146.6 °C; 294.3 to 295.8 °F; 418.9 to 419.7 K | ||
| Henry's law constant (kH) |
1.5 nmol Pa−1 kg−1 | ||
| Thermochemistry | |||
| 278.2 J K−1 mol−1 | |||
| Std molar entropy (S |
333.4 J K−1 mol−1 | ||
| Std enthalpy of combustion (ΔcH |
−6.1261–−6.1229 MJ mol−1 | ||
| Hazards | |||
| NFPA 704 | |||
| Related compounds | |||
| Related alkanes |
|||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Tetraethylmethane is a branched alkane with 9 carbon atoms. It is a highly flammable and volatile liquid at room temperature. It is one of the isomers of nonane.
References
- ↑ "Tetraethylmethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 11 March 2012.
See also
External links
This article is issued from Wikipedia - version of the 7/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.


