Technetium hexafluoride
 | |
| Identifiers | |
|---|---|
| 13842-93-8 | |
| Properties | |
| TcF6 | |
| Molar mass | 212 g/mol (98Tc) |
| Appearance | golden-yellow crystals[1] |
| Density | 3,58 g/cm3 (−140 °C), solid[2] |
| Melting point | 37.4 °C (99.3 °F; 310.5 K)[1] |
| Boiling point | 55.3 °C (131.5 °F; 328.4 K)[1] |
| Structure | |
| cubic | |
| Hazards | |
| EU classification (DSD) |
not listed |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961.[3] In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. The other such compound is technetium(VI) chloride, TcCl6. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7.[4] Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium.
Preparation
Technetium hexafluoride is prepared by heating technetium metal with an excess of F2 at 400 °C.[3]
- Tc + 3 F
2 → TcF
6
Description
Technetium hexafluoride is a golden-yellow solid at room temperature. Its melting point is 37.4 °C and its boiling point is 55.3 °C.[1]
Technetium hexafluoride undergoes a solid phase transition at −4.54 °C. Above this temperature (measured at 10 °C), the solid structure is cubic. Lattice parameters are a = 6.16 Å. There are two formula units (in this case, discrete molecules) per unit cell, giving a density of 3.02 g·cm−3. Below this temperature (measured at −19 °C), the solid structure is orthorhombic space group Pnma. Lattice parameters are a = 9.55 Å, b = 8.74 Å, and c = 5.02 Å. There are four formula units (in this case, discrete molecules) per unit cell, giving a density of 3.38 g·cm−3. At −140 °C, the solid structure is still orthothombic, but the lattice parameters are now a = 9.360 Å, b = 8.517 Å, and c = 4.934 Å, giving a density of 3.58 g·cm−3.[2]
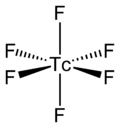
The TcF6 molecule itself (the form important for the liquid or gas phase) has octahedral molecular geometry, which has point group (Oh). The Tc–F bond length is 1.812 Å.[2] Its magnetic moment has been measured to be 0.45 μB.[5]
Properties
Physical
TcF6 is octahedral, as shown by infrared and Raman spectra.[6][7] Its low-temperature orthorhombic form converts to the higher symmetry body-centred cubic form at room temperature, like other metal hexafluorides such as RhF6 and OsF6.[8] Preliminary measurements of magnetic moment yield a value of 0.45 µB, which is lower than expected for a d1 octahedral compound.[9]
Chemical
TcF6 reacts with alkaline chlorides in iodine pentafluoride (IF5) solution to form hexafluorotechnetates.[10][11] TcF6 disproportionates on hydrolysis with aqueous NaOH to form a black precipitate of TcO2.[3] In hydrogen fluoride solution, TcF6 reacts with hydrazinium fluoride to yield N2H6TcF6 or N2H6(TcF6)2.[12]
References
- 1 2 3 4 CRC Handbook of Chemistry and Physics, 90th Edition, CRC Press, Boca Raton, Florida, 2009, ISBN 978-1-4200-9084-0, Section 4, Physical Constants of Inorganic Compounds, p. 4-93.
- 1 2 3 Drews, T.; Supeł, J.; Hagenbach, A.; Seppelt, K. (2006). "Solid State Molecular Structures of Transition Metal Hexafluorides". Inorganic Chemistry. 45 (9): 3782–3788. doi:10.1021/ic052029f. PMID 16634614.
- 1 2 3 Selig, H.; Chernick, C.L.; Malm, J.G. (1961). "The Preparation and Properties of TcF6". Journal of Inorganic and Nuclear Chemistry. 19 (3–4): 377–381.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Selig, H.; Cafasso, F. A.; Gruen, D. M.; Malm, J. G. (1962). "Magnetic Susceptibility of ReF6". Journal of Chemical Physics. 36 (12): 3440. Bibcode:1962JChPh..36.3440S. doi:10.1063/1.1732477.
- ↑ Howard H. Claassen; Henry Selig & John G. Malm (1962). "Vibrational Spectra of MoF6 and TcF6". Journal of Chemical Physics. 36 (11): 2888–2890. Bibcode:1962JChPh..36.2888C. doi:10.1063/1.1732396.
- ↑ Howard H. Claassen; Gordon L. Goodman; John H. Holloway & Henry Selig (1970). "Raman Spectra of MoF6, TcF6, ReF6, UF6, SF6, SeF6, and TeF6 in the Vapor State". Journal of Chemical Physics. 53 (1): 341–348. Bibcode:1970JChPh..53..341C. doi:10.1063/1.1673786.
- ↑ Siegel S & Northrop DA (1966). "X-Ray Diffraction Studies of Some Transition Metal Hexafluorides". Inorganic Chemistry. 5 (12): 2187–2188. doi:10.1021/ic50046a025.
- ↑ Selig, H; Cafasso, F A.; Gruen, D M.; Malm, J G. (1962). "Magnetic Susceptibility of ReF6". Journal of Chemical Physics. 36 (12): 3440–3444. Bibcode:1962JChPh..36.3440S. doi:10.1063/1.1732477.
- ↑ Edwards, A. J.; Hugill, D.; Peacock, R. D. (1963). "New Fluorine Compounds of Technetium". Nature. 200 (4907): 672. Bibcode:1963Natur.200..672E. doi:10.1038/200672a0.
- ↑ D. Hugill & R. D. Peacock (1966). "Some quinquevalent fluorotechnetates". Journal of the Chemical Society A: 1339–1341. doi:10.1039/J19660001339.
- ↑ Frlec B; Selig H & Hyman H.H (1967). "Hydrazinium(+2) Hexafluorometalates(IV) and -(V) in the 4d and 5d Transition Series". Inorganic Chemistry. 6 (10): 1775–1783. doi:10.1021/ic50056a004.