Table of thermodynamic equations
| Thermodynamics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
The classical Carnot heat engine | ||||||||||||
|
Branches |
||||||||||||
|
||||||||||||
| Book:Thermodynamics | ||||||||||||
This article is a summary of common equations and quantities in thermodynamics (see thermodynamic equations for more elaboration). SI units are used for absolute temperature, not Celsius or Fahrenheit.
Definitions
Many of the definitions below are also used in the thermodynamics of chemical reactions.
General basic quantities
Quantity (Common Name/s) (Common) Symbol/s SI Units Dimension Number of molecules N dimensionless dimensionless Number of moles n mol [N] Temperature T K [Θ] Heat Energy Q, q J [M][L]2[T]−2 Latent Heat QL J [M][L]2[T]−2
General derived quantities
Quantity (Common Name/s) (Common) Symbol/s Defining Equation SI Units Dimension Thermodynamic beta, Inverse temperature β J−1 [T]2[M]−1[L]−2 Thermodynamic Temperature τ J [M] [L]2 [T]−2 Entropy S ,
J K−1 [M][L]2[T]−2 [Θ]−1 Pressure P Pa M L−1T−2 Internal Energy U J [M][L]2[T]−2 Enthalpy H J [M][L]2[T]−2 Partition Function Z dimensionless dimensionless Gibbs free energy G J [M][L]2[T]−2 Chemical potential (of component i in a mixture)
μi , where F is not proportional to N because μi depends on pressure. , where G is proportional to N (as long as the molar ratio composition of the system remains the same) because μi depends only on temperature and pressure and composition.
J [M][L]2[T]−2 Helmholtz free energy A, F J [M][L]2[T]−2 Landau potential, Landau Free Energy, Grand potential Ω, ΦG J [M][L]2[T]−2 Massieu Potential, Helmholtz free entropy Φ J K−1 [M][L]2[T]−2 [Θ]−1 Planck potential, Gibbs free entropy Ξ J K−1 [M][L]2[T]−2 [Θ]−1
Thermal properties of matter
Quantity (common name/s) (Common) symbol/s Defining equation SI units Dimension General heat/thermal capacity C J K −1 [M][L]2[T]−2 [Θ]−1 Heat capacity (isobaric) Cp J K −1 [M][L]2[T]−2 [Θ]−1 Specific heat capacity (isobaric) Cmp J kg−1 K−1 [L]2[T]−2 [Θ]−1 Molar specific heat capacity (isobaric) Cnp J K −1 mol−1 [M][L]2[T]−2 [Θ]−1 [N]−1 Heat capacity (isochoric/volumetric) CV J K −1 [M][L]2[T]−2 [Θ]−1 Specific heat capacity (isochoric) CmV J kg−1 K−1 [L]2[T]−2 [Θ]−1 Molar specific heat capacity (isochoric) CnV J K −1 mol−1 [M][L]2[T]−2 [Θ]−1 [N]−1 Specific latent heat L J kg−1 [L]2[T]−2 Ratio of isobaric to isochoric heat capacity, heat capacity ratio, adiabatic index γ dimensionless dimensionless
Thermal transfer
Quantity (common name/s) (Common) symbol/s Defining equation SI units Dimension Temperature gradient No standard symbol K m−1 [Θ][L]−1 Thermal conduction rate, thermal current, thermal/heat flux, thermal power transfer P W = J s−1 [M] [L]2 [T]−3 Thermal intensity I W m−2 [M] [T]−3 Thermal/heat flux density (vector analogue of thermal intensity above) q W m−2 [M] [T]−3
Equations
The equations in this article are classified by subject.By Danish
Phase transitions
Physical situation Equations Adiabatic transition Isothermal transition For an ideal gas
Isobaric transition p1 = p2, p = constant
Isochoric transition V1 = V2, V = constant
Adiabatic expansion
Free expansion Work done by an expanding gas Process
Net Work Done in Cyclic Processes
Kinetic theory
Ideal gas equations Physical situation Nomenclature Equations Ideal gas law - p = pressure
- V = volume of container
- T = temperature
- n = number of moles
- R = Gas constant
- N = number of molecules
- k = Boltzmann's constant
Pressure of an ideal gas - m = mass of one molecule
- Mm = molar mass
Ideal gas
Quantity General Equation Isobaric
Δp = 0Isochoric
ΔV = 0Isothermal
ΔT = 0Adiabatic Work
WHeat Capacity
C(as for real gas)
(for monatomic ideal gas)
(for diatomic ideal gas)
(for monatomic ideal gas)
(for diatomic ideal gas)Internal Energy
ΔUEnthalpy
ΔHEntropy
Δs
[1]Constant
Entropy
- , where kB is the Boltzmann constant, and Ω denotes the volume of macrostate in the phase space or otherwise called thermodynamic probability.
- , for reversible processes only
Statistical physics
Below are useful results from the Maxwell–Boltzmann distribution for an ideal gas, and the implications of the Entropy quantity. The distribution is valid for atoms or molecules constituting ideal gases.
Physical situation Nomenclature Equations Maxwell–Boltzmann distribution - v = velocity of atom/molecule,
- m = mass of each molecule (all molecules are identical in kinetic theory),
- γ(p) = Lorentz factor as function of momentum (see below)
- Ratio of thermal to rest mass-energy of each molecule:
K2 is the Modified Bessel function of the second kind.
Non-relativistic speeds
Relativistic speeds (Maxwell-Jüttner distribution)
Entropy Logarithm of the density of states - Pi = probability of system in microstate i
- Ω = total number of microstates
where:
Entropy change
Entropic force Equipartition theorem - df = degree of freedom
Average kinetic energy per degree of freedom Internal energy
Corollaries of the non-relativistic Maxwell–Boltzmann distribution are below.
Physical situation Nomenclature Equations Mean speed Root mean square speed Modal speed Mean free path - σ = Effective cross-section
- n = Volume density of number of target particles
- ℓ = Mean free path
Quasi-static and reversible processes
For quasi-static and reversible processes, the first law of thermodynamics is:
where δQ is the heat supplied to the system and δW is the work done by the system.
Thermodynamic potentials
The following energies are called the thermodynamic potentials,
| Name | Symbol | Formula | Natural variables |
|---|---|---|---|
| Internal energy | |||
| Helmholtz free energy | |||
| Enthalpy | |||
| Gibbs free energy | |||
| Landau Potential (Grand potential) | , |
and the corresponding fundamental thermodynamic relations or "master equations"[2] are:
Potential Differential Internal energy Enthalpy Helmholtz free energy Gibbs free energy
Maxwell's relations
The four most common Maxwell's relations are:
Physical situation Nomenclature Equations Thermodynamic potentials as functions of their natural variables
More relations include the following.
Other differential equations are:
Name H U G Gibbs–Helmholtz equation
Quantum properties
- Indistinguishable Particles
where N is number of particles, h is Planck's constant, I is moment of inertia, and Z is the partition function, in various forms:
Degree of freedom Partition function Translation Vibration Rotation - where:
- σ = 1 (heteronuclear molecules)
- σ = 2 (homonuclear)
Thermal properties of matter
Coefficients Equation Joule-Thomson coefficient Compressibility (constant temperature) Coefficient of thermal expansion (constant pressure) Heat capacity (constant pressure) Heat capacity (constant volume) Derivation of heat capacity (constant pressure) Since
Derivation of heat capacity (constant volume) Since
(where δWrev is the work done by the system),
Thermal transfer
Physical situation Nomenclature Equations Net intensity emission/absorption - Texternal = external temperature (outside of system)
- Tsystem = internal temperature (inside system)
- ε = emmisivity
Internal energy of a substance - CV = isovolumetric heat capacity of substance
- ΔT = temperature change of substance
Meyer's equation - Cp = isobaric heat capacity
- CV = isovolumetric heat capacity
- n = number of moles
Effective thermal conductivities - λi = thermal conductivity of substance i
- λnet = equivalent thermal conductivity
Series Parallel
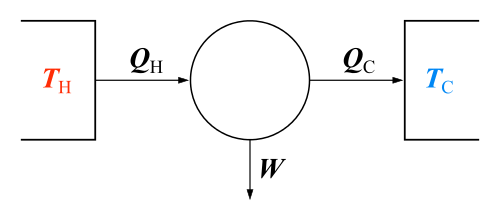
Thermal efficiencies
Physical situation Nomenclature Equations Thermodynamic engines - η = efficiency
- W = work done by engine
- QH = heat energy in higher temperature reservoir
- QL = heat energy in lower temperature reservoir
- TH = temperature of higher temp. reservoir
- TL = temperature of lower temp. reservoir
Thermodynamic engine:
Carnot engine efficiency:
Refrigeration - K = coefficient of refrigeration performance
Refrigeration performance Carnot refrigeration performance
See also
References
- ↑ Keenan, Thermodynamics, Wiley, New York, 1947
- ↑ Physical chemistry, P.W. Atkins, Oxford University Press, 1978, ISBN 0 19 855148 7
- Atkins, Peter and de Paula, Julio Physical Chemistry, 7th edition, W.H. Freeman and Company, 2002 [ISBN 0-7167-3539-3].
- Chapters 1 - 10, Part 1: Equilibrium.
- Bridgman, P.W., Phys. Rev., 3, 273 (1914).
- Landsberg, Peter T. Thermodynamics and Statistical Mechanics. New York: Dover Publications, Inc., 1990. (reprinted from Oxford University Press, 1978).
- Lewis, G.N., and Randall, M., "Thermodynamics", 2nd Edition, McGraw-Hill Book Company, New York, 1961.
- Reichl, L.E., "A Modern Course in Statistical Physics", 2nd edition, New York: John Wiley & Sons, 1998.
- Schroeder, Daniel V. Thermal Physics. San Francisco: Addison Wesley Longman, 2000 [ISBN 0-201-38027-7].
- Silbey, Robert J., et al. Physical Chemistry. 4th ed. New Jersey: Wiley, 2004.
- Callen, Herbert B. (1985). "Thermodynamics and an Introduction to Themostatistics", 2nd Ed., New York: John Wiley & Sons.