Staudinger synthesis
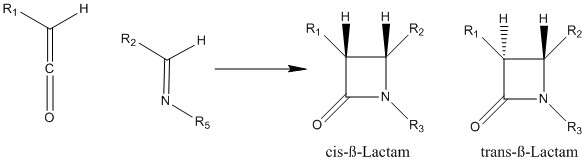
The Staudinger Synthesis, also called the Staudinger Ketene-Imine Cycloaddition, is a chemical synthesis in which an imine reacts with a ketene through a non-photochemical 2+2 cycloaddition to produce a β-lactam.[1] The reaction carries particular importance in the synthesis of β-Lactam antibiotics.[2] The Staudinger Synthesis should not be confused with the Staudinger Reaction, a phosphine or phosphite reaction used to reduce azides to amines. Reviews on the mechanism, stereochemistry, and applications of the reaction have been published.[3][4][5]
.jpg)
History
The reaction was discovered in 1907 by the German chemist Hermann Staudinger.[6] The reaction did not attract interest until the 1940s, when the structure of penicillin was elucidated. The β-lactam moiety of the first synthetic penicillin was constructed using this cycloaddition,[7] and it remains a valuable tool in synthetic organic chemistry.
Mechanism
.jpg)
The first step is a nucleophilic attack by the imine nitrogen on the carbonyl carbon to generate a zwitterionic intermediate. Electron donating groups on the imine facilitate this step, while electron-withdrawing groups impede the attack.[8] The second step is either an intramolecular nucleophilic ring closure or a conrotary electrocyclic ring closure.[9] The second step is different from typical electrocyclic ring closures as predicted by the Woodward–Hoffmann rules. Under photochemical and microwave conditions the intermediate's 4π-electron system cannot undergo a disrotatory ring closure to form the β-lactam, possibly because the two double bonds are not coplanar.[10] Some products of the Staudinger Synthesis differ from those predicted by the torquoelectronic model.[11] In addition, the electronic structure of the transition state differs from that of other conrotary ring closures.[11]
There is evidence from computational studies on model systems that in the gas phase the mechanism is concerted.[5]
Stereochemistry
The stereochemistry of the Staudinger Synthesis can be difficult to predict because either step can be rate-determining.[12] If the ring closure step is rate-determining, stereochemical predictions based on torquoselectivity are reliable.[12] Other factors that affect the stereochemistry include the initial regiochemistry of the imine. Generally, (E)-imines form cis β-lactams while (Z)-imines form trans β-lactams.[5] Other substituents affect the stereochemistry as well. Ketenes with strong electron-donating substituents mainly produce cis β-lactams, while ketenes with strong electron-withdrawing substituents generally produce trans β-lactams. The ketene substituent affects the transition state by either speeding up or slowing down the progress towards the β-lactam. A slower reaction allows for the isomerization of the imine, which generally results in a trans product.[11]
Variations
Reviews on asymmetric induction of the Staudinger Synthesis, including the use of organic and organometallic catalysts, have been published.[1][5][13]
The imine can be replaced by adding olefin to produce a cyclobutanone, carbonyl to produce a β-lactone, or carbodiimides to produce 4-imino β-lactams.[1] The Staudinger Synthesis and variations are all ketene cycloadditions.

In 2014, Doyle and coworkers reported a one-pot, multicomponent Staudinger synthesis of β-lactams from azides and two diazo compounds. The reaction occurs by a rhodium acetate-catalyzed reaction between the aryldiazoacetate (red) and the organic azide (blue) to form an imine. A Wolff Rearrangement of the diazoacetoacetate enone (pink) forms a stable ketene, which reacts with the imine to form a stable β-lactam compound.[14]

The reaction with sulfenes instead of ketenes leading to β-sultams is called Sulfa-Staudinger cycloaddition.[15]
References
- 1 2 3 Wright, Stephen in Corey, edited by Jie Jack Li ; foreword by E.J. (2010). Name reactions for carbocyclic ring formations. Hoboken, N.J.: Wiley. p. 45. ISBN 9780470872208.
- ↑ Tidwell, T. T. (2008). "Hugo (Ugo) Schiff, Schiff Bases, and a Century of β-Lactam Synthesis". Angew. Chem. Int. Ed. 47 (6): 1016–1020. doi:10.1002/anie.200702965. PMID 18022986.
- ↑ Fu, N.; Tidwell, T. T. "Preparation of β-lactams by [2+2] cycloaddition of ketenes and imines" Tetrahedron 2008, 64, 10465-10496. ()
- ↑ Georg, Gunda I. (1992). Organic Chemistry of β-Lactams. New York: Verlag Chemie. ISBN 0471187992.
- 1 2 3 4 Cossio, F. P.; Arrieta, A.; Sierra, M. G. (2008). "The Mechanism of the Ketene-Imine (Staudinger) Reaction in Its Centennial: Still an Unsolved Problem?". Accounts of Chemical Research. 41 (8): 925–936. doi:10.1021/ar800033j.
- ↑ H. Staudinger (1907). "Zur Kenntniss der Ketene. Diphenylketen". Justus Liebigs Ann. Chem. 356: 51–123. doi:10.1002/jlac.19073560106.
- ↑ J.C. Sheehan, E.L. Buhle, E.J. Corey, G.D. Laubach, J.J. Ryan (1950). "The Total Synthesis of a 5-Phenyl Penicillin: Methyl 5-Phenyl-(2-Carbomethoxyethyl)-Penicillinate". J. Am. Chem. Soc. 72 (8): 3828–9. doi:10.1021/ja01164a534.
- ↑ Wright, Stephen in Corey, edited by Jie Jack Li ; foreword by E.J. (2010). Name reactions for carbocyclic ring formations. Hoboken, N.J.: Wiley. p. 47. ISBN 9780470872208.
- ↑ Qi, Hengzhen; Li, Xinyao; Xu, Jiaxi (December 2010). "Stereoselective control in the Staudinger reactions involving monosubstituted ketenes with electron acceptor substituents: experimental investigation and theoretical rationalization". Organic and Biomolecular Chemistry. Royal Society of Chemistry. 9: 2702–2714. doi:10.1039/C0OB00783H.
- ↑ Liang, Yong; Jiao, Lei; Zhang, Shiwei; Xu, Jiaxi (2005). "Microwave- and Photoirradiation-Induced Staudinger Reactions of Cyclic Imines and Ketenes Generated from α-Diazoketones. A Further Investigation into the Stereochemical Process". Journal of Organic Chemistry. American Chemical Society. 70 (1): 334–337. doi:10.1021/jo048328o.
- 1 2 3 Jiao, Lei; Liang, Yong; Xu, Jiaxi (2006). "Origin of the Relative Stereoselectivity of the β-Lactam Formation in the Staudinger Reaction". Journal of American Chemical Society. American Chemical Society. 128 (18): 6060–6069. doi:10.1021/ja056711k.
- 1 2 Liang, Yong; Jiao, Lei; Zhang, Shiwei; Yu, Zhi-Xiang; Xu, Jiaxi (2009). "New Insights into the Torquoselectivity of the Staudinger Reaction". Journal of the American Chemical Society. American Chemical Society. 131 (4): 1542–1549. doi:10.1021/ja808046e.
- ↑ Palomo, Claudio; Aizpurua, Jesus M.; Ganboa, Iñaki; Oiarbide, Mikel (1999). "Asymmetric Synthesis of β-Lactams by Staudinger Ketene-Imine Cycloaddition Reaction". European Journal of Organic Chemistry. Wiley-VCH. 1999 (12): 3223–3235. doi:10.1002/(SICI)1099-0690(199912)1999:12<3223::AID-EJOC3223>3.0.CO;2-1.
- ↑ Mandler, Michael D.; Truong, Phong M.; Zavalij, Peter Y.; Doyle, Michael P. (2014). "Catalytic Conversion of Diazocarbonyl Compounds to Imines". Organic Letters. American Chemical Society. 16 (3): 740–743. doi:10.1021/ol403427s.
- ↑ Yang, Zhanhui; Chen, Ning; Xu, Jiaxi (2015). "Substituent-Controlled Annuloselectivity and Stereoselectivity in the Sulfa-Staudinger Cycloadditions". The Journal of Organic Chemistry. 80 (7): 3611–3620. doi:10.1021/acs.joc.5b00312. ISSN 0022-3263.