Rhodococcus
| Rhodococcus | |
|---|---|
 | |
| Rhodococcus sp. | |
| Scientific classification | |
| Kingdom: | Bacteria |
| Phylum: | Actinobacteria |
| Order: | Actinomycetales |
| Suborder: | Corynebacterineae |
| Family: | Nocardiaceae |
| Genus: | Rhodococcus Zopf 1891 |
Rhodococcus is a genus of aerobic, nonsporulating, nonmotile Gram-positive bacteria closely related to Mycobacterium and Corynebacterium.[1][2] While a few species are pathogenic, most are benign, and have been found to thrive in a broad range of environments, including soil, water, and eukaryotic cells. Fully sequenced in October 2006, the genome is known to be 9.7 megabasepairs long and 67% G/C.[3]
Strains of Rhodococcus are important owing to their ability to catabolize a wide range of compounds and produce bioactive steroids, acrylamide, and acrylic acid, and their involvement in fossil fuel biodesulfurization.[3] This genetic and catabolic diversity is not only due to the large bacterial chromosome, but also to the presence of three large linear plasmids.[1] Rhodococcus is also an experimentally advantageous system owing to a relatively fast growth rate and simple developmental cycle, but is not well characterized.[3]
Another important application of Rhodococcus comes from bioconversion, using biological systems to convert cheap starting material into more valuable compounds, such as its ability to metabolize harmful environmental pollutants, including toluene, naphthalene, herbicides, and PCBs. Rhodococcus species typically metabolize aromatic substrates by first oxygenating the aromatic ring to form a diol (two alcohol groups). Then, the ring is cleaved with intra/extradiol mechanisms, opening the ring and exposing the substrate to further metabolism. Since the chemistry is very stereospecific, the diols are created with predictable chirality. While controlling the chirality of chemical reaction presents a significant challenge for synthetic chemists, biological processes can be used instead to faithfully produce chiral molecules in cases where direct chemical synthesis is not feasible or efficient. An example of this is the use of Rhodococcus to produce indene, a precursor to the AIDS drug indinavir, a protease inhibitor, and containing two of the five chiral centers needed in the complex.[4]
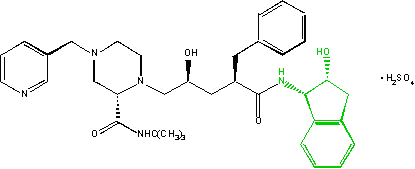
Biodegradation of organic pollutants
The burgeoning amount of bacterial genomic data provides unparalleled opportunities for understanding the genetic and molecular bases of the microbial biodegradation of organic pollutants. Aromatic compounds are among the most recalcitrant of these pollutants, and lessons can be learned from the recent genomic studies of R. jostii RHA1, one of the largest bacterial genomes completely sequenced to date. These studies have helped expand the understanding of bacterial catabolism, noncatabolic physiological adaptation to organic compounds, and the evolution of large bacterial genomes. A large number of "peripheral aromatic" pathways funnel a range of natural and xenobiotic compounds into a restricted number of "central aromatic" pathways. Some pathways are more widespread than initially thought. The Box and Paa pathways illustrate the prevalence of nonoxygenolytic ring-cleavage strategies in aerobic aromatic degradation processes. Functional genomic studies have been useful in establishing that even organisms harboring high numbers of homologous enzymes apparently contain few examples of true redundancy. For example, the multiplicity of ring-cleaving dioxygenases in certain rhodococcal isolates may be attributed to the cryptic aromatic catabolism of different terpenoids and steroids. The large gene repertoires of pollutant degraders such as R. jostii RHA1 have evolved principally through more ancient processes.[5] Rhodococcus sp. strain Q1 (American Type Culture Collection strain number 49987), isolated from soil and paper mill sludge, is able to degrade quinoline, various pyridine derivatives, catechol, benzoate, and protocatechuic acid.[6]
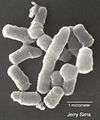 Scanning electron micrograph of Rhodococcus sp. strain Q1 grown on quinoline - the organism can use quinoline as a sole source of carbon, nitrogen, and energy, tolerating concentrations up to 3.88 millimoles per liter.
Scanning electron micrograph of Rhodococcus sp. strain Q1 grown on quinoline - the organism can use quinoline as a sole source of carbon, nitrogen, and energy, tolerating concentrations up to 3.88 millimoles per liter.
Pathogenic Rhodococcus
The genus Rhodococcus has two pathogenic species: R. fascians and R. equi. The former, a plant pathogen, causes leafy gall disease in both angiosperm and gymnosperm plants.[7] R. equi is the causative agent of foal pneumonia (rattles) and mainly infects foals up to three months in age. However, it has a wide host range, sporadically infecting pigs, cattle, and immunocompromised humans, in particular AIDS patients and those undergoing immunosuppressive therapy.[8] Interestingly, both pathogens rely on a conjugative virulence plasmid to cause disease. In case of R. fascians, this is a linear plasmid, whereas R. equi harbors a circular plasmid. Both pathogens are economically significant. R. fascians is a major pathogen of tobacco plants. R. equi, one of the most important foal pathogens, is endemic on many stud farms around the world.
In molecular biology
Rhodococcus has also been identified as a contaminant of DNA extraction kit reagents and ultrapure water systems, which may lead to its erroneous appearance in microbiota or metagenomic datasets.[9]
Species
- Rhodococcus aerolatus Hwang et al. 2015[10]
- Rhodococcus aetherivorans Goodfellow et al. 2004[10]
- Rhodococcus agglutinans Guo et al. 2015[10]
- Rhodococcus aurantiacus (ex Tsukamura and Mizuno, 1971) Tsukamura and Yano, 1985, nom. rev.
- Rhodococcus artemisiae Zhao et al. 2012[10]
- Rhodococcus baikonurensis Li, et al., 2004
- Rhodococcus biphenylivorans Su et al. 2015[10]
- Rhodococcus boritolerans
- Rhodococcus equi (Magnusson, 1923) Goodfellow and Alderson, 1977
- Rhodococcus canchipurensis Nimaichand et al. 2013[10]
- Rhodococcus cerastii Kämpfer et al. 2013[10]
- Rhodococcus cercidiphylli Li et al. 2012[10]
- Rhodococcus coprophilus Rowbotham and Cross, 1979
- Rhodococcus corynebacterioides (Serrano, et al., 1972) Yassin and Schaal, 2005 (synonym: Nocardia corynebacterioides (Serrano et al. 1972)
- Rhodococcus defluvii Kämpfer et al. 2014[10]
- Rhodococcus enclensis Dastager et al. 2014[10]
- Rhodococcus erythropolis (Gray and Thornton, 1928) Goodfellow and Alderson, 1979
- Rhodococcus fascians (Tilford 1936) Goodfellow 1984 (synonym: Rhodococcus luteus (ex Söhngen 1913) Nesterenko et al. 1982)[11]
- Rhodococcus globerulus Goodfellow, et al., 1985
- Rhodococcus gordoniae Jones, et al., 2004
- Rhodococcus hoagii Kämpfer et al. 2014[10]
- Rhodococcus imtechensis Ghosh et al. 2006[10]
- Rhodococcus jialingiae Wang et al. 2010[10]
- Rhodococcus jostii Takeuchi, et al., 2002. Identified as producing a lignin digesting enzyme, it was the first isolated from a bacterium rather than a fungus.[12][13]
- Rhodococcus koreensis Yoon, et al., 2000
- Rhodococcus kroppenstedtii Mayilraj, et al., 2006
- Rhodococcus kunmingensis Wang et al. 2008[10]
- Rhodococcus kyotonensis Li et al. 2007[10]
- Rhodococcus maanshanensis Zhang, et al., 2002
- Rhodococcus marinonascens Helmke and Weyland, 1984
- Rhodococcus nanhaiensis
- Rhodococcus opacus Klatte, et al., 1995
- Rhodococcus percolatus Briglia, et al., 1996
- Rhodococcus phenolicus Rehfuss and Urban, 2006
- Rhodococcus polyvorum Li et al. 2012[10]
- Rhodococcus pyridinivorans Yoon, et al., 2000
- Rhodococcus qingshengii Xu et al. 2007[10]
- Rhodococcus rhodochrous (Zopf 1891) Tsukamura, 1974
- Rhodococcus rhodnii Goodfellow and Alderson, 1979 (synonym: Nocardia rhodnii)
- Rhodococcus ruber (Kruse 1896) Goodfellow and Alderson, 1977 (synonym: Streptothrix rubra Kruse, 1896)
- Rhodococcus jostii RHA1
- Rhodococcus soli Li et al. 2015[10]
- Rhodococcus triatomae Yassin, 2005
- Rhodococcus trifolii Kämpfer et al. 2013[10]
- Rhodococcus tukisamuensis Matsuyama, et al., 2003
- Rhodococcus wratislaviensis (Goodfellow et al. 1995) Goodfellow, et al., 2002 (synonym: Tsukamurella wratislaviensis Goodfellow, et al., 1995)
- Rhodococcus yunnanensis Zhang, et al., 2005
- Rhodococcus zopfii Stoecker, et al., 1994
References
- 1 2 van der Geize R. & L. Dijkhuizen (2004). "Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications". Microbiology. 7 (3): 255–261. doi:10.1016/j.mib.2004.04.001. PMID 15196492.
- ↑ Burkovski A (editor). (2008). Corynebacteria: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-30-1. .
- 1 2 3 McLeod MP, Warren RL, Hsiao WW, Araki N, Myhre M, Fernandes C, Miyazawa D, Wong W, Lillquist AL, Wang D, Dosanjh M, Hara H, Petrescu A, Morin RD, Yang G, Stott JM, Schein JE, Shin H, Smailus D, Siddiqui AS, Marra MA, Jones SJ, Holt R, Brinkman FS, Miyauchi K, Fukuda M, Davies JE, Mohn WW, Eltis LD (October 17, 2006). "The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse". PNAS. 103 (42): 15582–15587. doi:10.1073/pnas.0607048103. PMC 1622865
 . PMID 17030794.
. PMID 17030794. - 1 2 Treadway, S.L., K.S. Yanagimachi, E. Lankenau, P.A. Lessard, G. Stephanopoulos and A.J. Sinskey (1999). "Isolation and characterization of indene bioconversion genes from Rhodococcus strain I24". Appl. Microbiol. Biotechnol. 51 (6): 786–793. doi:10.1007/s002530051463. PMID 10422226.
- ↑ McLeod MP & Eltis LD (2008). "Genomic Insights Into the Aerobic Pathways for Degradation of Organic Pollutants". Microbial Biodegradation: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-17-2.
- ↑ O'Loughlin, E.J.; Kehrmeyer, S.R.; Sims, G.K. (1996). "Isolation, characterization, and substrate utilization of a quinoline degrading bacterium". International Biodeterioration and Biodegradation. 38 (2): 107–118. doi:10.1016/S0964-8305(96)00032-7.
- ↑ Goethals, K.; Vereecke, D.; Jaziri, M.; Van, Montagu M.; Holsters, M. (2001). "Leafy gall formation by Rhodococcus fascians". Annu.Rev.Phytopathol. 39: 27–52. doi:10.1146/annurev.phyto.39.1.27. PMID 11701858.
- ↑ Muscatello, G.; Leadon, D. P.; Klay, M.; Ocampo-Sosa, A.; Lewis, D. A.; Fogarty, U.; Buckley, T.; Gilkerson, J. R.; Meijer, W. G.; et al. (2007). "Rhodococcus equi infection in foals: the science of 'rattles'". Equine Vet.J. 39 (5): 470–478. doi:10.2746/042516407x209217. PMID 17910275.
- ↑ Salter, S; Cox, M; Turek, E; Calus, S; Cookson, W; Moffatt, M; Turner, P; Parkhill, J; Loman, N; Walker, A (2014). "Reagent contamination can critically impact sequence-based microbiome analyses". BioRxiv. doi:10.1101/007187.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Parte, A.C. "Rhodococcus". www.bacterio.net.
- ↑ Klatte, S.; et al. (1994). "Rhodococcus luteus is a later subjective synonym of Rhodococcus fascians". Int. J. Syst. Bacteriol. 44 (4): 627–630. doi:10.1099/00207713-44-4-627.
- ↑ http://www.physorg.com/news/2011-06-wood-digesting-enzyme-bacteria-boost-biofuel.html
- ↑ Takeuchi, M; Hatano, K; Sedlácek, I; Pácová, Z (2002). "Rhodococcus jostii sp. nov., isolated from a medieval grave". International Journal of Systematic and Evolutionary Microbiology. 52 (Pt 2): 409–13. doi:10.1099/00207713-52-2-409. PMID 11931149.
External links
- For species and synonyms see here: National Center for Biotechnology Information (NCBI)