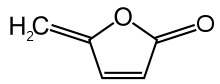
Protoanemonin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5-Methylidenefuran-2-one | |
| Other names
4-Methylenebut-2-en-4-olide | |
| Identifiers | |
| 108-28-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 60307 |
| ECHA InfoCard | 100.003.244 |
| PubChem | 66948 |
| UNII | 66FQZ1A5SO |
| |
| |
| Properties | |
| C5H4O2 | |
| Molar mass | 96.08 g/mol |
| Appearance | Pale yellow oil |
| Boiling point | 45 °C (113 °F; 318 K) 2 hPa |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
190 mg·kg−1 (mouse)[2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Protoanemonin (sometimes called anemonol or ranunculol[3]) is a toxin found in all plants of the buttercup family (Ranunculaceae). When the plant is wounded or macerated, the unstable glucoside found in the plant, ranunculin, is enzymatically broken down into glucose and the toxic protoanemonin.[4] It is the lactone of 4-hydroxy-2,4-pentadienoic acid.
Contact with a wounded plant causes itch, rashes or blistering on contact with the skin or mucosa. Ingesting the toxin can cause nausea, vomiting, dizziness, spasms, acute hepatitis, jaundice, or paralysis.[5][6][7]
When drying the plant, protoanemonin comes into contact with air and dimerizes to anemonin, which is further hydrolyzed to a non-toxic carboxylic acid.[4][8]
Biological pathway
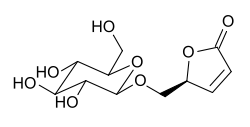 | ranunculin |
| ↓ – glucose | (maceration, enzymatically) |
 | protoanemonin |
| ↓ dimerization | (air or water contact) |
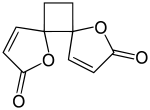 | anemonin |
| ↓ hydrolyzation | |
 |
References
- ↑ Römpp, Hermann; Falbe, Jürgen; Regitz, Manfred (1992). Römpp Lexikon Chemie (in German) (9 ed.). Stuttgart: Georg Thieme Verlag.
- ↑ Martín, ML; San Román, L; Domínguez, A (1990). "In vitro activity of protoanemonin, an antifungal agent.". Planta Medica. 56 (1): 66–9. doi:10.1055/s-2006-960886. PMID 2356244.
The LD50 of protoanemonin in male Swiss albino mice was 190 mg/kg.
- ↑ List, PH; Hörhammer, L, eds. (1979). Hagers Handbuch der pharmazeutischen Praxis (in German) (4 ed.). Springer Verlag. ISBN 3-540-07738-3.
- 1 2 Berger, Artur; Wachter, Helmut, eds. (1998). Hunnius Pharmazeutisches Wörterbuch (in German) (8 ed.). Walter de Gruyter Verlag. ISBN 3-11-015793-4.
- ↑ Yilmaz, Bulent; Yilmaz, Barış; Aktaş, Bora; Unlu, Ozan; Roach, Emir Charles (2015-02-27). "Lesser celandine (pilewort) induced acute toxic liver injury: The first case report worldwide". World Journal of Hepatology. 7 (2): 285–288. doi:10.4254/wjh.v7.i2.285. ISSN 1948-5182. PMC 4342611
 . PMID 25729484.
. PMID 25729484. - ↑ Verbraucherschutz, Bundesamt für (2014-09-12). List of Substances of the Competent Federal Government and Federal State Authorities: Category "Plants and plant parts". Springer. ISBN 9783319107325.
- ↑ Lewis, Robert Alan (1998-03-23). Lewis' Dictionary of Toxicology. CRC Press. ISBN 9781566702232.
- ↑ Handbuch der organischen Chemie, Leopold Gmelin (German)
This article is issued from Wikipedia - version of the 9/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.