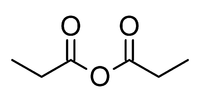
Propionic anhydride
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Propanoic anhydride | |
| Other names
Propionic anhydride Propanoyl propanoate | |
| Identifiers | |
| 123-62-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 29003 |
| ECHA InfoCard | 100.004.218 |
| PubChem | 31263 |
| RTECS number | UF9100000 |
| |
| |
| Properties | |
| C6H10O3 | |
| Molar mass | 130.14 g/mol |
| Appearance | Clear liquid, with a strong smell similar to vinegar |
| Density | 1.015 g/cm3, liquid |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 167 to 170 °C (333 to 338 °F; 440 to 443 K) |
| Reacts to give propanoic acid | |
| Viscosity | 1.144 cP at ?°C |
| Hazards | |
| Main hazards | flammable |
| Safety data sheet | External MSDS |
| R-phrases | R34 |
| S-phrases | S26-45 |
| Flash point | 63 °C (145 °F; 336 K) |
| Related compounds | |
| Related compounds |
Acetic anhydride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Propanoic anhydride is an organic compound with the formula (CH3CH2CO)2O. This simple acid anhydride is a colourless liquid. It is a widely used reagent in organic synthesis.
Synthesis
Propanoic anhydride has been prepared by dehydration of propanoic acid using ketene:[1]
- 2 CH3CH2CO2H + CH2=C=O → (CH3CH2CO)2O + CH3CO2H
Safety
Propanoic anhydride is strong smelling and corrosive, and will cause burns on contact with skin. Vapour can burn eyes and lungs.
Legal Status
Due to its potential use as a precursor in the synthesis of fentanyl and fentanyl analogs, propanoic anhydride is regulated by the United States Drug Enforcement Administration as a List I chemical under the Controlled Substances Act.[2]
References
- ↑ Williams, J. W. Krynitsky, J. A. (1955). "n-Caproic Anhydride". Org. Synth.; Coll. Vol., 3
- ↑ Drugs of Abuse Publication, Chapter 2
This article is issued from Wikipedia - version of the 9/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.