Folliculogenesis
- Although the process is similar in many animals, this article will deal exclusively with human folliculogenesis.

1 - Menstruation
2 - Developing follicle
3 - Mature follicle
4 - Ovulation
5 - Corpus luteum
6 - Deterioration of corpus luteum
In biology, folliculogenesis is the maturation of the ovarian follicle, a densely packed shell of somatic cells that contains an immature oocyte. Folliculogenesis describes the progression of a number of small primordial follicles into large preovulatory follicles that enter the menstrual cycle.
Contrary to male spermatogenesis, which can last indefinitely, folliculogenesis ends when the remaining follicles in the ovaries are incapable of responding to the hormonal cues that previously recruited some follicles to mature. This depletion in follicle supply signals the beginning of menopause.
Overview
The primary role of the follicle is oocyte support. From birth, the ovaries of the human female contain a number of immature, primordial follicles. These follicles each contain a similarly immature primary oocyte. After puberty and commencing with the first menstruation, a clutch of follicles begins folliculogenesis, entering a growth pattern that will end in death or in ovulation (the process where the oocyte leaves the follicle).
During post-pubescent follicular development, and over the course of roughly a year, primordial follicles that have begun development undergo a series of critical changes in character, both histologically and hormonally. Two-thirds of the way through this process, the follicles have transitioned to tertiary, or antral, follicles. At this stage in development, they become dependent on hormones emanating from the host body, causing a substantial increase in their growth rate.
With a little more than ten days until the end of the period of follicular development, most of the original group of follicles have died (a process known as atresia). The remaining cohort of follicles enter the menstrual cycle, competing with each other until only one follicle is left. This remaining follicle, the late tertiary or pre-ovulatory follicle, ruptures and discharges the oocyte (that has since grown into a secondary oocyte), ending folliculogenesis.
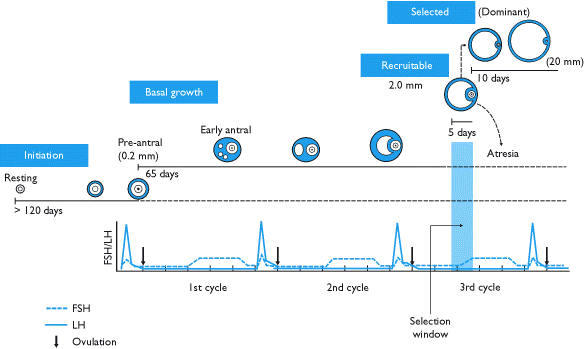
Phases of development
Folliculogenesis lasts for approximately 375 days. It coincides with thirteen menstrual cycles. The process begins continuously, meaning that at any time the ovary contains follicles in all stages of development, and ends when a mature oocyte departs from the preovulatory follicle in a process called ovulation.
The growing follicle passes through the following distinct stages that are defined by certain structural characteristics (unfamiliar terms will be defined in their respective sections):
In a larger perspective, the whole folliculogenesis, from primordial to preovulatory follicle, belongs to the stage of ootidogenesis of oogenesis.
| Stage | Description | Size |
| Primordial | Dormant, small, only one layer of flat granulosa cells | Primordial follicles are about 0.03-0.05 mm in diameter. |
| Primary | Mitotic cells, cuboidal granulosa cells | Almost 0.1 mm in diameter |
| Secondary | Presence of theca cells, multiple layers of granulosa cells | The follicle is now 0.2 mm in diameter |
| Early tertiary | The early tertiary follicle is arbitrarily divided into five classes. Class 1 follicles are 0.2 mm in diameter, class 2 about 0.4 mm, class 3 about 0.9 mm, class 4 about 2 mm, and class 5 about 5 mm. | |
| Late tertiary | Fully formed antrum, no further cytodifferentiation, no novel progress | Class 6 follicles are about 10 mm in diameter, class 7 about 16 mm, and class 8 about 20 mm. It is common for non-dominant follicles to grow beyond class 5, but rarely is there more than one class 8 follicle. |
| Preovulatory | Building growth in estrogen concentration, all other follicles atretic or dead |
In addition, follicles that have formed an antrum are called antral follicles or Graafian follicles. Definitions differ in where this shift occurs in the staging given above, with some stating that it occurs when entering the secondary stage,[1] and others stating that it occurs when entering the tertiary stage.[2]
Until the preovulatory stage, the follicle contains a primary oocyte that is arrested in prophase of meiosis I. During the late preovulatory stage, the oocyte continues meiosis and becomes a secondary oocyte, arrested in metaphase II.
Primordial
At 18–22 weeks post-conception, the cortex of the female ovary contains its peak number of follicles (about 4 to 5 million in the average case, but individual peak populations range from 6 to 7 million;[3] 'These primordial follicles contain immature oocytes surrounded by flat, squamous granulosa cells (the support cells) that are segregated from the oocyte's environment by the basal lamina. They are quiescent, showing little to no biological activity. Because primordial follicles can be dormant for up to 50 years in the human, the length of the ovarian cycle does not include this time.
The supply of follicles decreases slightly before birth, and to 180,000 by puberty for the average case (populations at puberty range from 25,000 to 1.5 million).[3] By virtue of the "inefficient" nature of folliculogenesis (discussed later), only 400 of these follicles will ever reach the preovulatory stage. At menopause, only 1,000 follicles remain. It seems likely that early menopause occurs for women with low populations at birth, and late menopause occurs for women with high populations at birth, but there is as yet no clinical evidence for this.[3]
The process by which primordial cells 'wake up' is known as initial recruitment. Research has shown that initial recruitment is mediated by the counterbalance of various stimulatory and inhibitory hormones and locally produced growth factors.[4]
Primary
The granulosa cells of these primordial follicles change from a flat to a cuboidal structure, marking the beginning of the primary follicle. The oocyte genome is activated and genes become transcribed. Rudimentary paracrine signalling pathways that are vital for communication between the follicle and oocyte are formed. Both the oocyte and the follicle grow dramatically, increasing to almost 0.1 mm in diameter.
Primary follicles develop receptors to follicle stimulating hormone (FSH) at this time, but they are gonadotropin-independent until the antral stage. Research has shown, however, that the presence of FSH accelerates follicle growth in vitro.
A glycoprotein polymer capsule called the zona pellucida forms around the oocyte, separating it from the surrounding granulosa cells. The zona pellucida, which remains with the oocyte after ovulation, contains enzymes that catalyze with sperm to allow penetration.
Secondary
Stroma-like theca cells are recruited by oocyte-secreted signals. They surround the follicle's outermost layer, the basal lamina, and undergo cytodifferentiation to become the theca externa and theca interna. An intricate network of capillary vessels forms between these two thecal layers and begins to circulate blood to and from the follicle.
The late-term secondary follicle is marked histologically and structurally by a fully grown oocyte surrounded by a zona pellucida, approximately nine layers of granulosa cells, a basal lamina, a theca interna, a capillary net, and a theca externa.
Antrum formation
The formation of a fluid-filled cavity adjacent to the oocyte called the antrum designates the follicle as an antral follicle, in contrast to so a called preantral follicle that still lacks an antrum. An antral follicle is also called a Graafian follicle.
Definitions differ in which stage this shift occurs, with some designating follicles in the secondary stage as antral,[1] and others designating them as preantral.[2]
Early tertiary
In the tertiary follicle, the basic structure of the mature follicle has formed and no novel cells are detectable. Granulosa and theca cells continue to undergo mitotis concomitant with an increase in antrum volume. Tertiary follicles can attain a tremendous size that is hampered only by the availability of FSH, which it is now dependent on.
Under action of an oocyte-secreted morphogenic gradient, the granulosa cells of the tertiary follicle undergo differentiation into four distinct subtypes: corona radiata, surrounding the zona pellucida; membrana, interior to the basal lamina; periantral, adjacent to the antrum and cumulus oophorous, which connects the membrana and corona radiata granulosa cells together. Each type of cell behaves differently in response to FSH.
Theca cells express receptors for luteinizing hormone (LH). LH induces the production of androgens by the theca cells, most notably androstendione, which are aromatized by granulosa cells to produce estrogens, primarily estradiol. Consequently, estrogen levels begin to rise.
Late tertiary and preovulatory (the follicular phase of the menstrual cycle)
At this point, the majority of the group of follicles that started growth 360 days ago have already died. This process of follicle death is known as atresia, and it is characterized by radical apoptosis of all constituent cells and the oocyte. Although it is not known what causes atresia, the presence of high concentrations of FSH has been shown to prevent it.
A rise in pituitary FSH caused by the disintegration of the corpus luteum at the conclusion of the twelfth menstrual cycle precipitates the selection of five to seven class 5 follicles to participate in the thirteenth. These follicles enter the end of the twelfth menstrual cycle and transition into the follicular phase of the thirteenth cycle. The selected follicles, called antral follicles, compete with each other for growth-inducing FSH.
In response to the rise of FSH, the antral follicles begin to secrete estrogen and inhibin, which have a negative feedback effect on FSH.[5] Follicles that have fewer FSH-receptors will not be able to develop further; they will show retardation of their growth rate and become atretic. Eventually, only one follicle will be viable. This remaining follicle, called the dominant follicle, will grow quickly and dramatically—up to 20 mm in diameter—to become the preovulatory follicle.
Note: Many sources misrepresent the pace of follicle growth, some even suggesting that it takes only fourteen days for a primordial follicle to become preovulatory. Actually, the follicular phase of the menstrual cycle means the time between selection of a tertiary follicle and its subsequent growth into a preovulatory follicle.The actual time for development of a follicle is varied among cases.
Ovulation and the corpus luteum
By the end of the follicular(or proliferative) phase of the thirteenth day of the menstrual cycle, the cumulus oophorus layer of the preovulatory follicle will develop an opening, or stigma, and excrete the oocyte with a complement of cumulus cells in a process called ovulation. The oocyte is technically still a secondary oocyte, suspended in the metaphase II of meiosis. It will develop into an ootid, and rapidly thereafter into an ovum (via completion of meiosis II) only upon fertilization. The oocyte will now travel down one of the fallopian tubes to eventually be discharged through menstruation in the case that it is unfertilized or if it is not successfully implanted in the uterus (if previously fertilized).
The ruptured follicle will undergo a dramatic transformation into the corpus luteum, a steroidiogenic cluster of cells that maintains the endometrium of the uterus by the secretion of large amounts of progesterone and minor amounts of estrogen.
These two steps, while not part of folliculogenesis, are included for completeness. They are discussed in their entirety by their respective articles, and placed into perspective by the menstrual cycle article. It is recommended that these three topics be reviewed.
Hormone function
As with most things related to the reproductive system, folliculogenesis is controlled by the endocrine system. Five hormones participate in an intricate process of positive and negative feedback to regulate folliculogenesis. They are:
- gonadotropin-releasing hormone (GnRH) secreted by the hypothalamus
- two gonadotropins:
- follicle-stimulating hormone (FSH)
- luteinizing hormone (LH)
- estrogen
- progesterone
GnRH stimulates the release of FSH and LH from the anterior pituitary gland that will later have a stimulatory effect on follicle growth (not immediately, however, because only antral follicles are dependent on FSH and LH). When theca cells form in the tertiary follicle the amount of estrogen increases sharply (theca-derived androgen is aromatized into estrogen by the granulosa cells).
At low concentration, estrogen inhibits gonadotropins, but high concentration of estrogen stimulates them. In addition, as more estrogen is secreted, more LH receptors are made by the theca cells, inciting theca cells to create more androgen that will become estrogen downstream. This positive feedback loop causes LH to spike sharply, and it is this spike that causes ovulation.
Following ovulation, LH stimulates the formation of the corpus luteum. Estrogen has since dropped to negative stimulatory levels after ovulation and therefore serves to maintain the concentration of FSH and LH. Inhibin, which is also secreted by the corpus luteum, contributes to FSH inhibition.
The endocrine system coincides with the menstrual cycle and goes through thirteen cycles (and thus thirteen LH spikes) during the course of normal folliculogenesis. However, coordinated enzyme signalling and the time-specific expression of hormonal receptors ensures that follicle growth does not become disregulated during these premature spikes.
Number of follicles
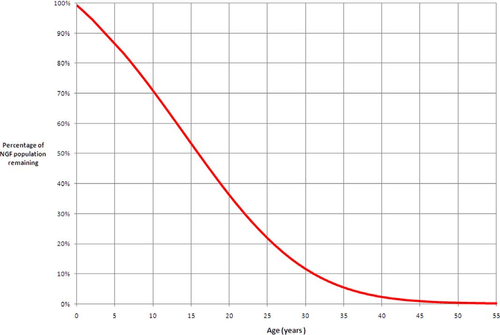
Recently, two publications have challenged the idea that a finite number of follicles are set around the time of birth.[7][8] Renewal of ovarian follicles from germline stem cells (originating from bone marrow and peripheral blood) was reported in the postnatal mouse ovary. Studies attempting to replicate these results are underway, but a study of populations in 325 human ovaries found no supporting evidence for follicular replenishment.[3]
In 2010, researchers at the University of Edinburgh determined that by the time women are 30 years old, only 10% of their non-growing follicles (NGFs) remain.[6] At birth, women have all their follicles for folliculogenesis, and they steadily decline until menopause.
Depletion of the ovarian reserve
As women (and mice) age, double-strand breaks accumulate in their primordial follicle reserve. These follicles contain primary oocytes that are arrested in prophase of the first cell division of meiosis. Double-strand breaks are accurately repaired during meiosis by searching for, and building off of, the matching strand (termed “homologous recombinational repair”). Titus et al.[9] (2013) found that, as humans (and mice) age, expression of four key DNA repair genes necessary for homologous recombinational repair declines in oocytes. They hypothesized that DNA double-strand break repair is vital for the maintenance of oocyte reserve, and that a decline in efficiency of repair with age plays a key role in the depletion of the ovarian reserve (ovarian aging).
See also
- ovarian follicle
- granulosa cells
- fertilization
- menstrual cycle
- ovulation
- reproductive cycle
- spermatogenesis
- Follicular atresia
- Oocyte maturation inhibitor
Additional images
-
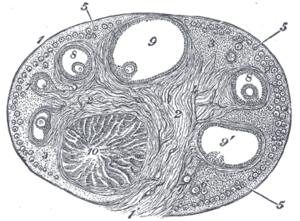
Section of the ovary. (#5 through #9 represent stages of folliculogenesis)
-
.jpg)
transitional primary follicle.
References
- 1 2 Page 769, section "formation of the antrum" in: Sherwood, Lauralee. (2010). Human physiology : from cells to system. Australia ; United States: Brooks/Cole. ISBN 978-0-495-39184-5.
- 1 2 Page 76 in: Vandenhurk, R.; Bevers, M.; Beckers, J. (1997). "In-vivo and in-vitro development of preantral follicles". Theriogenology. 47: 73–82. doi:10.1016/S0093-691X(96)00341-X.
- 1 2 3 4 Wallace WHB and Kelsey TW (2010) Human Ovarian Reserve from Conception to the Menopause. PLoS ONE 5(1):e8772. doi:10.1371/journal.pone.0008772
- ↑ Fortune J, Cushman R, Wahl C, Kito S (2000). "The primordial to primary follicle transition.". Mol Cell Endocrinol. 163 (1-2): 53–60. doi:10.1016/S0303-7207(99)00240-3. PMID 10963874.
- ↑ de Ziegler D (2007), "Roles of FSH and LH during the follicular phase: insight into the natural cycle IVF", RBM Online volume 15 No. 5, page 508
- 1 2 Wallace, W. Hamish B.; Thomas W. Kelsey (2010-01-27). "Human Ovarian Reserve from Conception to the Menopause". PLoS ONE. 5 (1): e8772. doi:10.1371/journal.pone.0008772. PMC 2811725
 . PMID 20111701.
. PMID 20111701. - ↑ Johnson J, Bagley J, Skaznik-Wikiel M, Lee H, Adams G, Niikura Y, Tschudy K, Tilly J, Cortes M, Forkert R, Spitzer T, Iacomini J, Scadden D, Tilly J (2005). "Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood.". Cell. 122 (2): 303–15. doi:10.1016/j.cell.2005.06.031. PMID 16051153.
- ↑ Johnson J, Canning J, Kaneko T, Pru J, Tilly J (2004). "Germline stem cells and follicular renewal in the postnatal mammalian ovary.". Nature. 428 (6979): 145–50. doi:10.1038/nature02316. PMID 15014492.
- ↑ Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. (2013). Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 5(172):172ra21. doi: 10.1126/scitranslmed.3004925. PMID 23408054
- Caglar G, Asimakopoulos B, Nikolettos N, Diedrich K, Al-Hasani S (2005). "Recombinant LH in ovarian stimulation.". Reprod Biomed Online. 10 (6): 774–85. doi:10.1016/S1472-6483(10)61123-6. PMID 15970010.
- Gougeon A (1996). "Regulation of ovarian follicular development in primates: facts and hypotheses.". Endocr Rev. 17 (2): 121–55. doi:10.1210/er.17.2.121. PMID 8706629.
- Gougeon A (1986). "Dynamics of follicular growth in the human: a model from preliminary results.". Hum Reprod. 1 (2): 81–7. PMID 3558758.
- van den Hurk R, Zhao J (2005). "Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles.". Theriogenology. 63 (6): 1717–51. doi:10.1016/j.theriogenology.2004.08.005. PMID 15763114.
External links
- Morphology and Physiology of the Ovary at endotext.org
- The ovary - folliculogenesis and oogenesis at nlm.nih.gov
- Folliculogenesis Graphic Depicting Hormones Involved at anshlabs.com
- Folliculogenesis and Ovulation at gfmer.ch
- Reproductive Physiology at ufp.pt