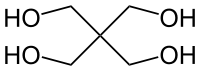
Pentaerythritol
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Bis(hydroxymethyl)propane-1,3-diol[1] | |
| Other names
2,2-Bis(hydroxymethyl)1,3-propanediol Pentaerythritol[1] Hercules P 6 Monopentaerythritol Tetramethylolmethane THME PETP Pentaerythrite Pentek Hercules Aqualon improved technical PE-200 | |
| Identifiers | |
| 115-77-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 7984 |
| ECHA InfoCard | 100.003.732 |
| UNII | SU420W1S6N |
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 |
| Appearance | white solid |
| Density | 1.396g/cm3 |
| Melting point | 260.5 °C (500.9 °F; 533.6 K) |
| Boiling point | 276 °C (529 °F; 549 K) at 30 mmHg |
| 5.6 g/100 mL at 15 °C | |
| Solubility | Soluble in methanol, ethanol, glycerol, ethylene glycol, formamide; insoluble in acetone, benzene, paraffin, ether, CCl4 |
| Vapor pressure | 0.00000008 mmHg (20°C)[2] |
| Hazards | |
| Flash point | 200.1 °C (392.2 °F; 473.2 K) |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[2] |
| REL (Recommended) |
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[2] |
| IDLH (Immediate danger) |
N.D.[2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Pentaerythritol is an organic compound, a white, crystalline solid with the formula C5H12O4. It is a polyol, and a sugar alcohol with the neopentane backbone and one hydroxyl group in each of the four terminal carbons. It is a building block for the synthesis and production of explosives, plastics, appliances, and many other important chemicals.
History
Pentaerythritol was first synthesized in 1891 by German chemist Bernhard Tollens and his student P. Wigand.[3]
Uses
Pentaerythritol is a versatile building block for the preparation of many polyfunctionalized compounds, such as the explosive and vasodilator pentaerythritol tetranitrate (PETN) and the polymer cross-linking agent pentaerythritol tetraacrylate.[4] Derivatives of pentaerythritol are components of alkyd resins, varnishes, polyvinyl chloride stabilizers, tall oil esters, and olefin antioxidants. Can be found in transformer oil, plastics, and many other applications.
Pentaerythritol is also used in the synthesis of Feneritrol.
Medicine
Pentaerythritol is used to synthesize vasodilator pentaerythritol tetranitrate (PETN).
Explosive
Pentaerythritol is used to synthesize explosive pentaerythritol tetranitrate (PETN).
Dielectric
Halogen-free pentaerythritol esters are also environmentally friendly alternative to conventional electrical transformer fluids, being both readily biodegradable and non-hazardous in water. They advantageously replace polychlorobiphenyl (PCB), and even silicone-based or fluorinated hydrocarbons, as dielectric fluid in transformers. Their low volatility and high flash point give them an excellent resistance to ignition in case of major electrical failure and transformer rupture.
Fire retardant
Pentaerythritol is used as a fire retardant, such as in plastics.[5]
Synthesis
Pentaerythritol can be synthesized by reacting 1 mole Acetaldehyde with 4 moles of Formaldehyde.
The reaction proceeds by substitution of two Alpha hydrogens of acetaldehyde by the reduced form of formaldehyde, i.e. Hydroxymethyl group.
This results in the formation of 3-Hydroxy-2-(hydroxymethyl)propanal The next step proceeds with the Cannizzaro between the above formed product and final mole of formaldehyde.
This forms the final product, pentaerythritol.
References
- 1 2 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0485". National Institute for Occupational Safety and Health (NIOSH).
- ↑ B. Tollens and P.Wigand (1891) "Ueber den Penta-Erythrit, einen aus Formaldehyd und Acetaldehyd synthetisch hergestellten vierwerthigen Alkohol" (On pentaerythritol, a quaternary alcohol synthetically produced from formaldehyde and acetaldehyde), Annalen der Chemie, 265 : 316-340.
- ↑ S. F. Marrian (1948). "The Chemical Reactions of Pentaerythritol and its Derivatives". Chemical Reviews. 43 (1): 149–202. doi:10.1021/cr60134a004. PMID 18876970.
- ↑ B. Pani, S. Sirohi, D. Singh (2013). "Studies on the Effects of Various Flame Retardants on Polypropylene". American Journal of Polymer Science.