Nitroapocynin
 | |
| Names | |
|---|---|
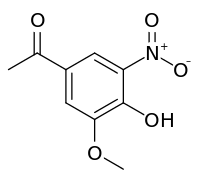
| Preferred IUPAC name
1-(4-Hydroxy-3-methoxy-5-nitrophenyl)ethan-1-one | |
| Other names
1-(4-hydroxy-3-methoxy-5-nitrophenyl)ethanone 4'-Hydroxy-3'-methoxy-5'-nitroacetophenone Nitroapocynin 5-Nitroapocynin | |
| Identifiers | |
| 20716-41-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 260051 |
| PubChem | 294764 |
| |
| |
| Properties | |
| C9H9NO5 | |
| Molar mass | 211.17 g·mol−1 |
| Appearance | Yellow powder |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitroapocynin is a mono-nitrated form of apocynin.
Synthesis
Apocynin can be nitrated with sodium nitrate and acidic ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate in acetonitrile solvent.[1][2]
References
- ↑ Tajik, H.; Niknam, K.; Parsa, F. (2009). "Using acidic ionic liquid 1-butyl-3-methylimidazolium hydrogen sulfate in selective nitration of phenols under mild conditions". Journal of the Iranian Chemical Society. 6: 159. doi:10.1007/BF03246515.
- ↑ Babu, Sainath; Raghavamenon, Achuthan C.; Fronczek, Frank R.; Uppu, Rao M. (2009). "4-Hydroxy-3-methoxy-5-nitroacetophenone (5-nitroapocynin)". Acta Crystallographica Section E. 65 (9): o2292. doi:10.1107/S160053680903390X.
This article is issued from Wikipedia - version of the 9/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.