Napthalenetetracarboxylic diimide
 Top: AFM image of NTCDI molecules on silver interacting via hydrogen bonding (the hydrogen bonds are exaggerated by artifacts of the imaging technique[1][2]); bottom: model (grey: C, white: H, red: O, blue: N)[3] | |
| Names | |
|---|---|
| Systematic IUPAC name
Benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetrone | |
| Other names
1,4,5,8-Naphthalenetetracarbondiimide | |
| Identifiers | |
| 5690-24-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 72067 |
| EC Number | 227-159-0 |
| |
| |
| Properties | |
| C14H6N2O4 | |
| Molar mass | 266.21 g·mol−1 |
| Melting point | > 300 °C[4] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Naphthalenetetracarboxylic diimide (NTCDI) is a solid organic compound and one of the simplest naphthalenediimides (NDIs). NTCDI is produced from the parent naphthalene via an intermediate compound naphthalenetetracarboxylic dianhydride.
Properties
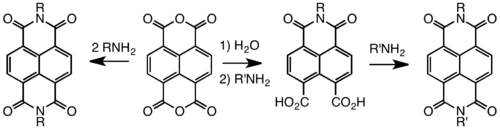
NTCDI is redox-active, forming stable radical anions near -1.10 V vs. Fc/Fc+.[5] Its ability to accept electrons reflects the presence of an extended conjugated ring system and the electron withdrawing groups (carbonyl centers). NDI is used in supramolecular chemistry owing to its tendency to form charge-transfer complexes with crown ethers, e.g., to give rotaxanes and catenanes. As another consequence of their planar structure and electron-acceptor properties, NDIs intercalate into DNA. It is also suitable for fabrication of soft electronic devices.[6]
References
- ↑ Hapala, Prokop; Kichin, Georgy; Wagner, Christian; Tautz, F. Stefan; Temirov, Ruslan; Jelínek, Pavel (2014-08-19). "Mechanism of high-resolution STM/AFM imaging with functionalized tips". Physical Review B. 90 (8): 085421. doi:10.1103/PhysRevB.90.085421.
- ↑ Hämäläinen, Sampsa K.; van der Heijden, Nadine; van der Lit, Joost; den Hartog, Stephan; Liljeroth, Peter; Swart, Ingmar (2014-10-31). "Intermolecular Contrast in Atomic Force Microscopy Images without Intermolecular Bonds". Physical Review Letters. 113 (18): 186102. doi:10.1103/PhysRevLett.113.186102. PMID 25396382.
- ↑ Sweetman, A. M.; Jarvis, S. P.; Sang, Hongqian; Lekkas, I.; Rahe, P.; Wang, Yu; Wang, Jianbo; Champness, N.R.; Kantorovich, L.; Moriarty, P. (2014). "Mapping the force field of a hydrogen-bonded assembly". Nature Communications. 5. doi:10.1038/ncomms4931.
- ↑ Lide, David R.; Milne, G.W.A. (1993). CRC Handbook of Data on Organic Compounds. CRC Press. p. 1522. ISBN 978-0-8493-0445-3.
- ↑ Bhosale, Sheshanath V.; Jani, Chintan H.; Langford, Steven J. (2008). "Chemistry of naphthalene diimides". Chem. Soc. Rev. 37 (2): 331. doi:10.1039/b615857a. PMID 18197349.
- ↑ Zhan, Xiaowei; Facchetti, Antonio; Barlow, Stephen; Marks, Tobin J.; Ratner, Mark A.; Wasielewski, Michael R.; Marder, Seth R. (2011). "Rylene and Related Diimides for Organic Electronics". Advanced Materials. 23 (2): 268. doi:10.1002/adma.201001402. PMID 21154741.