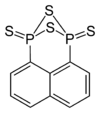
Naphthalen-1,8-diyl 1,3,2,4-dithiadiphosphetane 2,4-disulfide
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Naphthalen-1,8-diyl 1,3,2,4-dithiadiphosphetane 2,4-disulfide | |||
| Systematic IUPAC name
3,14-Dithia-2λ5,4λ5-diphosphatetracyclo[7.3.1. 12,4.05,13]tetradeca-1(12),5,7,9(13),10- | |||
| Identifiers | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 9419032 | ||
| PubChem | 11243996 | ||
| |||
| |||
| Properties | |||
| C10H6P2S4 | |||
| Molar mass | 316.36 g mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
A compound related to Lawesson's reagent named NpP2S4 has been formed by the reaction of 1-bromonaphthalene with P4S10, this is a 1,3,2,4-dithiadiphosphetane 2,4-disulfide which has a naphth-1,8-diyl group holding the two phosphorus atoms together. The mechanism by which the NpP2S4 forms is unclear, but it is thought to occur through some free radical process, and naphthalene has been detected as a side product in its synthesis. In general NpP2S4 has been found to be less reactive than Lawesson's reagent, this result agrees with the hypothesis that the dithiophosphine ylides are responsible for the majority of the chemical reactions of the 1,3,2,4-dithiadiphosphetane 2,4-disulfides.
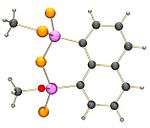
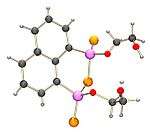
It has been found that NpP2S4 reacts with many hydroxyl compounds, for instance methanol, ethylene glycol and a catechol to form species with oxygen atoms bonded to the phosphorus atoms.
Methanol reacts to form compound which has one O-methyl and one S-methyl bonded to the two phosphorus atoms.
 |  |  |