Mannose phosphate isomerase
| Mannose-6 phosphate isomerase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 5.3.1.8 | ||||||||
| CAS number | 9023-88-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Mannose-6 phosphate isomerase (MPI), alternately phosphomannose isomerase (PMI) (EC 5.3.1.8) is an enzyme which facilitates the interconversion of fructose 6-phosphate(F6P) and mannose-6-phosphate(M6P). Mannose-6-Phosphate Isomerase may also enable the synthesis of GDP-mannose in eukaryotic organisms. M6P can be converted to F6P by Mannose-6-Phosphate Isomerase and subsequently utilized in several metabolic pathways including Glycolysis and capsular polysaccharide biosynthesis.[1] PMI is monomeric and metallodependent on Zinc as a cofactor ligand.[2] PMI is inhibited by erythrose 4-phosphate, mannitol 1-phosphate, and to a lesser extent, the alpha anomer of M6P.[3]
Mechanism
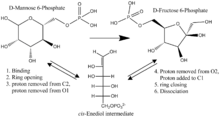
MPI must convert an aldose (mannose) to a ketose (fructose), in addition to opening and closing the rings for these sugars.[4] In humans a mechanism has been suggested which involves a hydrogen transfer between C1 and C2, mediated by Tyr278, and the movement of a proton from O1 and O2 mediated by the associated Zn2+ ion.[5] The ring opening step may be catalyzed by His99 and Asp270, and isomerization is likely a cis-enediol mechanism.[6] A detailed description of the mechanism of phosphomannose isomerase may be found in the 1968 Journal of Biological Chemistry paper written by Robert W. Gracy and Ernst A Noltmann titled "Studies on Phosphomannose Isomerase: A Mechanism for Catalysis and for the Role of Zinc in the Enzymatic and the Nonenzymatic Isomerization."[7]
PMI shows a high degree of selectivity for the beta anomer of M6P, and the alpha anomer has no activity, and may in fact act as an inhibitor.[8] Phosphoglucose Isomerase (PGI) has a very similar function to PMI, (as it catalyzes the interconversion of Glucose 6-Phosphate and F6P) however PGI can anomerize alpha and beta G6P, and may also catalyze the conversion of alpha M6P to beta M6P, while PMI may not anomerize M6P.[8] It is likely the cis-enediol intermediate formed by PMI is the same intermediate formed by PGI.[9]
Structure
MPI is composed of 440 Amino Acid residues, with one active site and one zinc ion Ligand. Amino acids GLN 111A, HIS 113A, GLU 138A, HIS 285A, and HOH 798A are involved with the zinc ligand bonding.[2] Structure differs from phosphoglucose isomerase by a threonine residue (Thr291) which creates extra space in the active site of PMI to accommodate the different stereochemistry of M6P. This increased space created by the threonine allows the rotation of the C2-C3 bond, which enables the necessary cis-enediol intermediate to be formed. Because mannose and glucose are stereoisomers at C2, which is crucial to the mechanism for both enzymes, PMI must allow extra space in the active site to allow for rotation of mannose to form the cis-enediol intermediate, which is the same intermediate formed by Phosphoglucose Isomerase.[10]
Biological Relevance
PMI has several contributions to necessary metabolic pathways. It enables cells to transform M6P into F6P, which can then be entered into Glycolysis. PMI also allows cells to convert F6P into M6P, which is a common glycolytic cellular identifier for cellular transport and cell membrane identification in prokaryotic and eukaryotic organisms.[4]
Medical Relevance
PMI may be helpful in the development of new antifungal treatments, as lack of PMI activity in yeast cells can lead to cell lysis and the enzyme may be a target for inhibition.[11] This may be due to the role of PMI in the formation cell walls and capsular polysaccharide biosynthesis. Additionally M6P is an important signaling molecule, especially for transport to lysosomes: disorders affecting MPI activity may affect cellular ability to quickly produce M6P from plentiful F6P, and therefore vesicle traffic to lysosomes and endosomes may be altered, potentially negatively impacting the cell.[12]
See also
External links
- GeneReviews/NCBI/NIH/UW entry on Congenital Disorders of Glycosylation Overview
- Mannose-6-Phosphate Isomerase at the US National Library of Medicine Medical Subject Headings (MeSH)
- http://www.ebi.ac.uk/interpro/IEntry?ac=IPR016305
Notes
- ↑ EBI Database, IPRO16305 Mannose-6-phosphate Isomerase.
- 1 2 PDBe 1pmi Summary.http://www.ebi.ac.uk/pdbe-srv/view/entry/1pmi/summary
- ↑ Gao, H., Yu, Y., Leary, JA. (2005). "Mechanism and Kinetics of metalloenzyme phosphomannose isomerase: measurement of dissociation constants and effect of zinc binding using ESI-FTICR mass spectrometry". Analytical Chemistry. 77 (17): 5596–603. doi:10.1021/ac050549m. PMID 16131071.
- 1 2 Berg, Tymoczko, Stryer (2007). Biochemistry (6th ed.). New York, New York: W.H. Freeman &Co.
- ↑ Xiao, J., Guo, Z., Guo, Y. Chu, F., Sun, P. (2006). "Computational study of human phosphomannose isomerase: Insights from homology modeling and molecular dynamics simulation of enzyme bound substrate.". J Mol Graph Model, Epub. 25: 289–95. doi:10.1016/j.jmgm.2006.01.001. PMID 16488169.
- ↑ Sagurthi, SR., Gowda G., Savithri HS., Murthy MR. (2009). "Structures of mannose-6-phosphate isomerase from Salmonella typhimurium bound to metal atoms and substrate: implications for catalytic mechanism.". Acta Crystallogr D. 65 (7): 724–32. doi:10.1107/S0907444909013328. PMID 19564693.
- ↑ Gracy, RW; Noltman E. (1968). "Studies on Phosphomannose Isomerase: A Mechanism for Catalysis and for the Role of Zinc in the Enzymatic and the Nonenzymatic Isomerization" (PDF). The Journal of Biological Chemistry. 243 (20): 5410–19.
- 1 2 Rose, I., O'Connell, E., Schray, K. (1973). "Mannose 6-Phosphate: Anomeric Form used by Phosphomannose Isomerase and Its 1-Epimerization by Phosphoglucose Isomerase" (PDF). The Journal of Biological Chemistry. 248 (6): 2232–34.
- ↑ Ruibo, W., Hujun, X., Zexing, C., Yirong, Mo. (2008). "Combined Quantum Mechanics/Molecular Mechanics Study on the Rversible Isomerization of Glucose and Fructose Catalyzed by Pyrococcus furiosus Phosphoglucose Isomerase. ePub" (PDF). J. AM. CHEM. SOC. 130 (22): 7022–31. doi:10.1021/ja710633c.
- ↑ Swan, M., Hansen, T., Schonheit, P., Davies, C. (2004). "Structural Basis for Phosphomannose Isomerase Activity in Phosphoglucose Isomerase from Pyrobaculum Aerophilum: A Subtle Difference between Distantly Related Enzymes". Biochemistry. 43: 14088–14095. doi:10.1021/bi048608y.
- ↑ Cleasby, Anne et al. (1996). "The X-ray crystal structure of phosphomannose isomerase from Candida albicans at 1.7 Å resolution". Nature Structural Biology. 3: 470–9. doi:10.1038/nsb0596-470.
- ↑ Jaeken, J., Matthijs, G. (2001). "Congenital Disorders of Glycosylation.". Annu. Rev. Genomics Hum. Genet. 2: 129–151. doi:10.1146/annurev.genom.2.1.129. PMID 11701646.