Leonurine
 | |
| Names | |
|---|---|
| IUPAC name
4-(Diaminomethylideneamino)butyl 4-hydroxy-3,5-dimethoxybenzoate | |
| Identifiers | |
| 24697-74-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 141828 |
| ECHA InfoCard | 100.208.686 |
| KEGG | C16985 |
| PubChem | 161464 |
| |
| |
| Properties | |
| C14H21N3O5 | |
| Molar mass | 311.34 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Leonurine is a pseudoalkaloid that has been isolated from Leonotis leonurus (Lion's tail), Leonotis nepetifolia, Leonotis artemisia, Leonurus cardiaca (Motherwort), Leonurus sibiricus, as well as other plants of family Lamiaceae. Leonurine is easily extracted into water.[1]
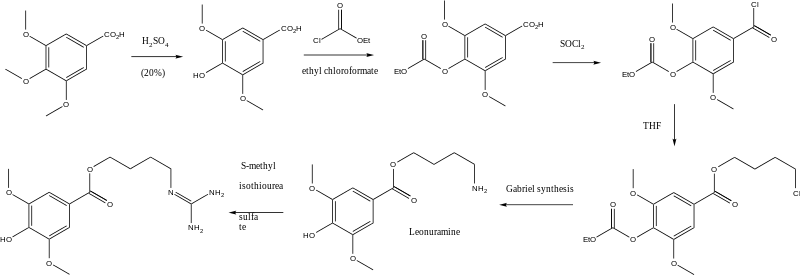
Chemical synthesis
Leonurine can be synthesized starting from eudesmic acid. Reaction with sulfuric acid produces syringic acid. Protection with ethyl chloroformate followed by reaction with thionyl chloride SOCl2 and then tetrahydrofuran yields 4-carboethoxysyringic acid 4-chloro-1-butyl ester. The chloride is then converted to an amino group via a Gabriel synthesis with potassium pthalimide) followed by hydrazinolysis (Ing–Manske procedure). The final step is reaction of the amine with S-methylisothiourea hemisulfate salt.[2]
References
- 1 2 "The Leonurine and its preparation". An Hui New Star Pharmaceutical Development Co. 2008. Retrieved 2008-08-28.
- ↑ "S-Methylisothiourea sulfate structure.". ChemSpider (RSC). 2014. Retrieved 2014-07-09.
Further reading
- Cheng KF, Yip CS, Yeung HW, Kong YC (May 1979). "Leonurine, an improved synthesis". Experientia. 35 (5): 571–2. doi:10.1007/BF01960323. PMID 446644.