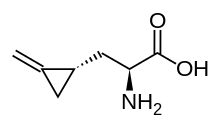
Hypoglycin A
 | |
| Names | |
|---|---|
| IUPAC name
(S)-2-Amino-3-((S)-2-methylenecyclopropyl)propanoic acid | |
| Other names
Hypoglycin A; Hypoglycine; 2-Methylenecyclopropanylalanine | |
| Identifiers | |
| 156-56-9 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1615355 |
| ChemSpider | 9943349 |
| ECHA InfoCard | 100.189.936 |
| PubChem | 11768666 |
| |
| |
| Properties | |
| C7H11NO2 | |
| Molar mass | 141.17 g·mol−1 |
| Melting point | 282 °C (540 °F; 555 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hypoglycin A is a naturally-occurring amino acid derivative found in the unripened fruit of the Ackee tree (Blighia sapida). It is toxic if ingested, and is the causative agent of Jamaican vomiting sickness.[1]
Sources
The entirety of the unripe Ackee fruit is toxic and contains large amounts of hypoglycin. The fruit is safe to eat only when the fruit is allowed to fully open and expose the large black seeds while on the tree. The levels of the toxin decrease over time though from approximately 1000 ppm to around 0.1 ppm in the mature fruit.[2]
Relatives of Ackee, including lychee, longan, and rambutan, can contain enough α-(methylenecyclopropyl)glycine, a homologue of hypoglycin A, in their fruit to cause hypoglycemic encephalopathy in undernourished children, when consumed in large quantities.[3]
Toxicity
Hypoglycin A is a type of Trojan Horse Inhibitor, also known as a Suicide Inhibitor, that in itself does not inhibit any biochemical pathway. However, when mistaken for a branch chain amino acid, the toxicity arises from its metabolites. The branched-chain alpha-keto acid dehydrogenase complex, that would normally convert luecine, isoleucine, or valine into Acyl-CoA derivates, takes this hypoglycin A and metabolizes it into toxic inhibitors; methylene cyclopropyl acetic acid and methylenecyclopropane acetyl esters with carnitine and coenzyme A. The FAD prosthetic group necessary for the beta oxidation of fatty acids associates with the alpha carbon of MCPA-CoA creating an irreversible complex that inhibits the activity of acyl CoA dehydrogenase. In addition, MCPA-CoA blocks some enzymes that are required for gluconeogenesis.[2]
The reduction in gluconeogenesis and the reduction in fatty acid oxidation are thought to be the cause of most of the symptoms of Jamaican vomiting sickness. The blocking of fatty acid metabolism causes cells to start using glycogen for energy. Once glycogen is depleted, the body is unable to produce more, which leads to a severe case of hypoglycemia. These biochemical effects are detected by an excess of medium chain fatty acids in urine and acidosis. Key treatments are aimed at circumventing or counteracting the biochemical changes, and include IV fluids and glucose, and hemodialysis in the case of renal failure.[4]
Synthesis
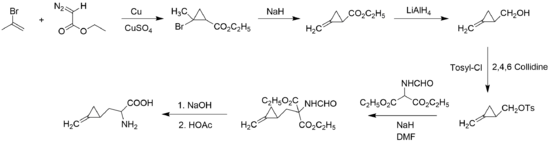
In 1958, John Carbon, William Martin, and Leo Swett were the first to synthesize hypoglycin, in racemic form, starting from 2-bromopropene and ethyl diazoacetate to form the cyclopropane ring.[5]
See also
References
- ↑ "Ackee Fruit Toxicity". Medscape.
- 1 2 "THE ACKEE FRUIT (BLIGHIA SAPIDA) AND ITS ASSOCIATED TOXIC EFFECTS". University of British Columbia.
- ↑ Spencer, P. S.; Palmer, V. S.; Mazumder, R. (2015), "Probable Toxic Cause for Suspected Lychee-Linked Viral Encephalitis", Emerging Infectious Diseases, 21, doi:10.3201/eid2105.141650
- ↑ "Hypoglycin". TOXNET.
- ↑ Carbon, J. A.; Martin, W. B.; Swett, L. R. (1958), "SYNTHESIS OF α-AMINO- METHYLENECYCLOPROPANEPROPIONIC ACID (HYPOGLYCIN A)", J. Am. Chem. Soc., 80: 1002–1002, doi:10.1021/ja01537a066