Ethyldichloroarsine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ethylarsonous dichloride | |||
| Other names
ED Dichloroethylarsane | |||
| Identifiers | |||
| 598-14-1 | |||
| ChemSpider | 11219 | ||
| ECHA InfoCard | 100.009.019 | ||
| PubChem | 11711 | ||
| Properties | |||
| C2H5AsCl2 | |||
| Molar mass | 174.8893 g/mol | ||
| Appearance | Colorless, mobile liquid | ||
| Density | 1.742 @ 14 deg C | ||
| Melting point | -65°C | ||
| Boiling point | -156°C (decomposes) | ||
| Soluble in alcohol, benzene, ether, and water | |||
| Hazards | |||
| Main hazards | Highly toxic, irritant | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
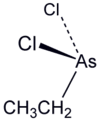
Ethyldichloroarsine, sometimes abbreviated "ED", is an organoarsenic compound with the formula CH3CH2AsCl2. This colourless volatile liquid is a highly toxic obsolete vesicant or blister agent that was used during World War I in chemical warfare.[1] The molecule is pyramidal with the Cl-As-Cl and C-As-Cl angles approaching 90° (see image). Its toxic action is similar to lewisite.
References
This article is issued from Wikipedia - version of the 11/14/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.