Ethanol (data page)
| |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Ethanol[1] | |||
| Other names
Absolute alcohol, alcohol, cologne spirit, drinking alcohol, ethane monoxide, ethylic alcohol, EtOH, ethyl alcohol, ethyl hydrate, ethyl hydroxide, ethylol, grain alcohol, hydroxyethane, methylcarbinol | |||
| Identifiers | |||
| 64-17-5 | |||
| 3D model (Jmol) | Interactive image | ||
| 3DMet | B01253 | ||
| 1718733 | |||
| ChEBI | CHEBI:16236 | ||
| ChEMBL | ChEMBL545 | ||
| ChemSpider | 682 | ||
| DrugBank | DB00898 | ||
| EC Number | 200-578-6 | ||
| 787 | |||
| 2299 | |||
| KEGG | D00068 | ||
| MeSH | Ethanol | ||
| PubChem | 702 | ||
| RTECS number | KQ6300000 | ||
| UNII | 3K9958V90M | ||
| UN number | 1170 | ||
| |||
| |||
| Properties | |||
| C2H6O | |||
| Molar mass | 46.068 g/mol[2] | ||
| Appearance | Colorless liquid | ||
| Density | 0.7893 g/cm3 (at 20 °C)[2] | ||
| Melting point | −114.14 ± 0.03[2] °C (−173.45 ± 0.05 °F; 159.01 ± 0.03 K) | ||
| Boiling point | 78.24 ± 0.09[2] °C (172.83 ± 0.16 °F; 351.39 ± 0.09 K) | ||
| miscible | |||
| log P | −0.18 | ||
| Vapor pressure | 5.95 kPa (at 20 °C) | ||
| Acidity (pKa) | 15.9 (H2O), 29.8 (DMSO)[3][4] | ||
| Basicity (pKb) | −1.9 | ||
| Refractive index (nD) |
1.3611[2] | ||
| Viscosity | 1.2 mPa·s (at 20 °C), 1.074 mPa·s (at 25 °C)[5] | ||
| 1.69 D[6] | |||
| Pharmacology | |||
| D08AX08 (WHO) V03AB16 (WHO), V03AZ01 (WHO) | |||
| Legal status |
| ||
| Moderate[9] | |||
| Moderate (10–15%)[10] | |||
| Common: oral Uncommon: suppository, inhalation, ocular, insufflation,[11] injection[12] | |||
| Pharmacokinetics: | |||
| Variable[13] | |||
| Hepatic enzyme alcohol dehydrogenase | |||
| Acetaldehyde, acetic acid, acetyl-CoA, carbon dioxide, water | |||
| None, constant rate elimination[14] | |||
| Urine, breath, perspiration, tears, milk, saliva, bile[14] | |||
| Hazards[15] | |||
| GHS pictograms |  [16] [16] | ||
| GHS signal word | Flammable | ||
| EU classification (DSD) |
| ||
| R-phrases | R11 | ||
| S-phrases | (S2), S7, S16 | ||
| NFPA 704 | |||
| Flash point | 16 °C (61 °F; 289 K) | ||
| 365 °C (689 °F; 638 K) | |||
| Explosive limits | 3.3%–19%[17] | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
7060 mg/kg (oral, rat)[18] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 1000 ppm (1900 mg/m3)[17] | ||
| REL (Recommended) |
TWA 1000 ppm (1900 mg/m3)[17] | ||
| IDLH (Immediate danger) |
3300 ppm[17] | ||
| Related compounds | |||
| Related compounds |
Ethane Methanol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
This page provides supplementary chemical data on ethanol. Except where noted otherwise, data relate to standard ambient temperature and pressure.
Material Safety Data Sheet
Structure and properties
| Structure and properties | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index of refraction, n25 | 1.361 | ||||||||||||||||||||||||||||||||||||||
| Dielectric constant, εr | 24.3 ε0 at 20 °C | ||||||||||||||||||||||||||||||||||||||
| Bond strength | ? | ||||||||||||||||||||||||||||||||||||||
| Bond length | ? | ||||||||||||||||||||||||||||||||||||||
| Bond angle | ? | ||||||||||||||||||||||||||||||||||||||
| Magnetic susceptibility[19] | 5.8·10−7 (cgs units, volume) | ||||||||||||||||||||||||||||||||||||||
| Surface tension | 22.39 dyn/cm at 25 °C | ||||||||||||||||||||||||||||||||||||||
| Viscosity[20] |
| ||||||||||||||||||||||||||||||||||||||
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 150 K (−123 °C), 0.00043 Pa |
| Critical point | 514 K (241 °C), 63 bar |
| Std enthalpy change of fusion, ΔfusH |
+4.9 kJ/mol |
| Std entropy change of fusion, ΔfusS |
+31 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
+38.56 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
109.67 J/(mol·K) |
| Molal freezing point constant | −1.99 °C kg/mol |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
−277.7 kJ/mol |
| Standard molar entropy, S |
160.7 J/(mol K)[21] |
| Heat capacity, cp | 111.46 J/(mol K) [21] |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−277.38 kJ/mol |
| Standard molar entropy, S |
159.9 J/(mol K) |
| Enthalpy of combustion, ΔcH |
−1370.7 kJ/mol |
| Heat capacity, cp | 112.4 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−235.3 kJ/mol |
| Standard molar entropy, S |
283 J/(mol K) |
| Heat capacity,[22][23] cp | 78.28 J/(mol K) at 90 °C 87.53 J/(mol K) at 110-220 °C |
| Heat capacity ratio,[22][23] γ = cp/cv |
1.13 at 90 °C |
| van der Waals' constants[24] | a = 1217.9 L2 kPa/mol2 b = 0.08407 L/mol |
Spectral data
| UV-Vis | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax | ? nm | ||||||||||||||||||||||||||||
| Extinction coefficient, ε | ? | ||||||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||||||
| Major absorption bands[25] |
| ||||||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||||||
| Proton NMR | |||||||||||||||||||||||||||||
| Carbon-13 NMR | http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/IMG.cgi?fname=CDS00245&imgdir=cdsW; | ||||||||||||||||||||||||||||
| Other NMR data | |||||||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||||||
| Masses of main fragments |
|||||||||||||||||||||||||||||
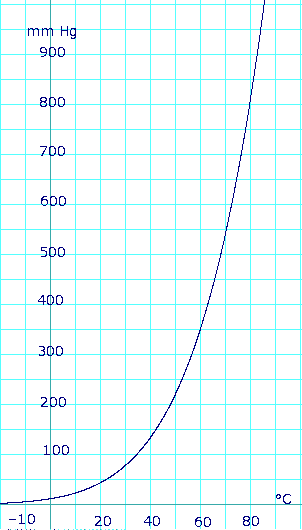
Vapor pressure of liquid
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Density of ethanol at various temperatures (kg/l or g/cm3)
Data obtained from Lange's Handbook of Chemistry, 10th ed.
| T | ρ | T | ρ | T | ρ |
| 3 °C | 0.80374 | 16 °C | 0.79283 | 29 °C | 0.78182 |
| 4 °C | 0.80290 | 17 °C | 0.79198 | 30 °C | 0.78097 |
| 5 °C | 0.80207 | 18 °C | 0.79114 | 31 °C | 0.78012 |
| 6 °C | 0.80123 | 19 °C | 0.79029 | 32 °C | 0.77927 |
| 7 °C | 0.80039 | 20 °C | 0.78945 | 33 °C | 0.77841 |
| 8 °C | 0.79956 | 21 °C | 0.78860 | 34 °C | 0.77756 |
| 9 °C | 0.79872 | 22 °C | 0.78775 | 35 °C | 0.77671 |
| 10 °C | 0.79788 | 23 °C | 0.78691 | 36 °C | 0.77585 |
| 11 °C | 0.79704 | 24 °C | 0.78606 | 37 °C | 0.77500 |
| 12 °C | 0.79620 | 25 °C | 0.78522 | 38 °C | 0.77414 |
| 39 °C | 0.77329 | 40 °C | 0.77244 |
These data correlate as Density (g/cm3) = -8.461834E-4 T( °C) + 0.8063372 with an R2 coefficient of determination of 0.99999.
Properties of aqueous ethanol solutions
Data obtained from Lange's Handbook of Chemistry, 10th ed. The annotation, d a °C/b °C, indicates density of solution at temperature a divided by density of pure water at temperature b.
| % wt ethanol | % vol ethanol | grams ethanol per 100 cc 15.56 °C | d 10 °C/4 °C | d 20 °C/4 °C | d 25 °C/4 °C | d 30 °C/4 °C | d 20 °C/20 °C | d 25 °C/25 °C | freezing temp. |
| 0.0 | 0.0 | 0.0 | 0.99973 | 0.99823 | 0.99708 | 0.99568 | 1.00000 | 1.00000 | 0 °C |
| 1.0 | 0.99785 | 0.99636 | 0.99520 | 0.99379 | 0.99813 | 0.99811 | |||
| 2.0 | 0.99602 | 0.99453 | 0.99336 | 0.99194 | 0.99629 | 0.99627 | |||
| 2.5 | 3.13 | 0.99363 | –1 °C | ||||||
| 3.0 | 0.99426 | 0.99275 | 0.99157 | 0.99014 | 0.99451 | 0.99447 | |||
| 4.0 | 5.00 | 3.97 | 0.99258 | 0.99103 | 0.98984 | 0.98839 | 0.99279 | 0.99274 | |
| 4.8 | 6.00 | 4.76 | 0.98971 | –2 °C | |||||
| 5.0 | 0.99098 | 0.98938 | 0.98817 | 0.98670 | 0.99113 | 0.99106 | |||
| 5.05 | 6.30 | 5.00 | 0.98930 | ||||||
| 6.0 | 0.98946 | 0.98780 | 0.98656 | 0.98507 | 0.98955 | 0.98945 | |||
| 6.8 | 8.47 | 0.98658 | –3 °C | ||||||
| 7.0 | 0.98801 | 0.98627 | 0.98500 | 0.98347 | 0.98802 | 0.98788 | |||
| 8.0 | 0.98660 | 0.98478 | 0.98346 | 0.98189 | 0.98653 | 0.98634 | |||
| 9.0 | 0.98524 | 0.98331 | 0.98193 | 0.98031 | 0.98505 | 0.98481 | |||
| 10.0 | 12.40 | 9.84 | 0.98393 | 0.98187 | 0.98043 | 0.97575 | 0.98361 | 0.98330 | |
| 11.0 | 0.98267 | 0.98047 | 0.97897 | 0.97723 | 0.98221 | 0.98184 | |||
| 11.3 | 14.0 | 11.11 | 0.98006 | –5 °C | |||||
| 12.0 | 0.98145 | 0.97910 | 0.97753 | 0.97573 | 0.98084 | 0.98039 | |||
| 13.0 | 0.98026 | 0.97775 | 0.97611 | 0.97424 | 0.97948 | 0.97897 | |||
| 13.78 | 17.00 | 13.49 | –6.1 °C | ||||||
| 14.0 | 0.97911 | 0.97643 | 0.97472 | 0.97278 | 0.97816 | 0.97757 | |||
| 15.0 | 0.97800 | 0.97514 | 0.97334 | 0.97133 | 0.97687 | 0.97619 | |||
| 15.02 | 18.50 | 14.68 | 0.97511 | ||||||
| 16.0 | 0.97692 | 0.97387 | 0.97199 | 0.96990 | 0.97560 | 0.97484 | |||
| 16.4 | 20.2 | 0.97336 | –7.5 °C | ||||||
| 17.0 | 0.97583 | 0.97259 | 0.97062 | 0.96844 | 0.97431 | 0.97346 | |||
| 17.5 | 21.5 | 0.97194 | –8.7 °C | ||||||
| 18.0 | 22.10 | 17.54 | 0.97473 | 0.97129 | 0.96923 | 0.96697 | 0.97301 | 0.97207 | |
| 18.8 | 23.1 | 0.97024 | –9.4 °C | ||||||
| 19.0 | 0.97363 | 0.96997 | 0.96782 | 0.96547 | 0.97169 | 0.97065 | |||
| 20.0 | 0.97252 | 0.96864 | 0.96639 | 0.96395 | 0.97036 | 0.96922 | |||
| 20.01 | 24.50 | 19.44 | 0.96863 | ||||||
| 20.3 | 24.8 | 0.96823 | –10.6 °C | ||||||
| 21.0 | 0.97139 | 0.96729 | 0.96495 | 0.96242 | 0.96901 | 0.96778 | |||
| 22.0 | 0.97024 | 0.96592 | 0.96348 | 0.96087 | 0.96763 | 0.96630 | |||
| 22.11 | 27.00 | 21.43 | 0.96578 | –12.2 °C | |||||
| 23.0 | 0.96907 | 0.96453 | 0.96199 | 0.95929 | 0.96624 | 0.96481 | |||
| 24.0 | 0.96787 | 0.96312 | 0.96048 | 0.95769 | 0.96483 | 0.96329 | |||
| 24.2 | 29.5 | 0.96283 | –14.0 °C | ||||||
| 25.0 | 30.40 | 24.12 | 0.96665 | 0.96168 | 0.95895 | 0.95607 | 0.96339 | 0.96176 | |
| 26.0 | 0.96539 | 0.96020 | 0.95738 | 0.95422 | 0.96190 | 0.96018 | |||
| 26.7 | 32.4 | 0.95914 | –16.0 °C | ||||||
| 27.0 | 0.96406 | 0.95867 | 0.95576 | 0.95272 | 0.96037 | 0.95856 | |||
| 28.0 | 33.90 | 26.90 | 0.96268 | 0.95710 | 0.95410 | 0.95098 | 0.95880 | 0.95689 | |
| 29.0 | 0.96125 | 0.95548 | 0.95241 | 0.94922 | 0.95717 | 0.95520 | |||
| 29.9 | 36.1 | 0.95400 | –18.9 °C | ||||||
| 30.0 | 36.20 | 28.73 | 0.95977 | 0.95382 | 0.95067 | 0.94741 | 0.95551 | 0.95345 | |
| 31.0 | 0.95823 | 0.95212 | 0.94890 | 0.94557 | 0.95381 | 0.95168 | |||
| 32.0 | 0.95665 | 0.95038 | 0.94709 | 0.94370 | 0.95207 | 0.94986 | |||
| 33.0 | 0.95502 | 0.94860 | 0.94525 | 0.94180 | 0.95028 | 0.94802 | |||
| 33.8 | 40.5 | 0.94715 | –23.6 °C | ||||||
| 34.0 | 0.95334 | 0.94679 | 0.94337 | 0.93986 | 0.94847 | 0.94613 | |||
| 35.0 | 0.95162 | 0.94494 | 0.94146 | 0.93790 | 0.94662 | 0.94422 | |||
| 35.04 | 41.90 | 33.25 | 0.94486 | ||||||
| 36.0 | 0.94986 | 0.94306 | 0.93952 | 0.93591 | 0.94473 | 0.94227 | |||
| 37.0 | 0.94805 | 0.94114 | 0.93756 | 0.93390 | 0.94281 | 0.94031 | |||
| 38.0 | 0.94620 | 0.93919 | 0.93556 | 0.93186 | 0.94086 | 0.93830 | |||
| 39.0 | 46.3 | 0.94431 | 0.93720 | 0.93353 | 0.92979 | 0.93886 | 0.93626 | –28.7 °C | |
| 40.0 | 0.94238 | 0.93518 | 0.93148 | 0.92770 | 0.93684 | 0.93421 | |||
| 40.04 | 47.40 | 37.61 | 0.93510 | ||||||
| 41.0 | 0.94042 | 0.93314 | 0.92940 | 0.92558 | 0.93479 | 0.93212 | |||
| 42.0 | 0.93842 | 0.93107 | 0.92729 | 0.92344 | 0.93272 | 0.93001 | |||
| 43.0 | 0.93639 | 0.92897 | 0.92516 | 0.92128 | 0.93062 | 0.92787 | |||
| 44.0 | 0.93433 | 0.92685 | 0.92301 | 0.91910 | 0.92849 | 0.92571 | |||
| 45.0 | 0.93226 | 0.92472 | 0.92085 | 0.91692 | 0.92636 | 0.92355 | |||
| 45.31 | 53.00 | 42.07 | 0.92406 | ||||||
| 46.0 | 0.93017 | 0.92257 | 0.91868 | 0.91472 | 0.92421 | 0.92137 | |||
| 46.3 | 53.8 | 0.92193 | –33.9 °C | ||||||
| 47.0 | 0.92806 | 0.92041 | 0.91649 | 0.91250 | 0.92204 | 0.91917 | |||
| 48.0 | 0.92593 | 0.91823 | 0.91429 | 0.91028 | 0.91986 | 0.91697 | |||
| 49.0 | 0.92379 | 0.91604 | 0.91208 | 0.90805 | 0.91766 | 0.91475 | |||
| 50.0 | 0.92162 | 0.91384 | 0.90985 | 0.90580 | 0.91546 | 0.91251 | |||
| 50.16 | 58.0 | 46.04 | 0.91349 | ||||||
| 51.0 | 0.91943 | 0.91160 | 0.90760 | 0.90353 | 0.91322 | 0.91026 | |||
| 52.0 | 0.91723 | 0.90936 | 0.90524 | 0.90125 | 0.91097 | 0.90799 | |||
| 53.0 | 0.91502 | 0.90711 | 0.90307 | 0.89896 | 0.90872 | 0.90571 | |||
| 54.0 | 0.91279 | 0.90485 | 0.90079 | 0.89667 | 0.90645 | 0.90343 | |||
| 55.0 | 0.91055 | 0.90258 | 0.89850 | 0.89437 | 0.90418 | 0.90113 | |||
| 55.16 | 63.0 | 50.00 | 0.90220 | ||||||
| 56.0 | 0.90831 | 0.90031 | 0.89621 | 0.89206 | 0.90191 | 0.89833 | |||
| 56.1 | 63.6 | 0.90008 | –41.0 °C | ||||||
| 57.0 | 0.90607 | 0.89803 | 0.89392 | 0.88975 | 0.89962 | 0.89654 | |||
| 58.0 | 0.90381 | 0.89574 | 0.89162 | 0.88744 | 0.89733 | 0.89423 | |||
| 59.0 | 0.90154 | 0.89344 | 0.88931 | 0.88512 | 0.89502 | 0.89191 | |||
| 60.0 | 0.89927 | 0.89113 | 0.88699 | 0.88278 | 0.89271 | 0.88959 | |||
| 60.33 | 68.0 | 53.98 | 0.89038 | ||||||
| 61.0 | 0.89898 | 0.88882 | 0.88466 | 0.88044 | 0.89040 | 0.88725 | |||
| 62.0 | 0.89468 | 0.88650 | 0.88233 | 0.87809 | 0.88807 | 0.88491 | |||
| 63.0 | 0.89237 | 0.88417 | 0.87998 | 0.87574 | 0.88574 | 0.88256 | |||
| 64.0 | 0.89006 | 0.88183 | 0.87763 | 0.87337 | 0.88339 | 0.88020 | |||
| 65.0 | 0.88774 | 0.87948 | 0.87527 | 0.87100 | 0.88104 | 0.87783 | |||
| 66.0 | 0.88541 | 0.87713 | 0.87291 | 0.86863 | 0.87869 | 0.87547 | |||
| 67.0 | 0.88308 | 0.87477 | 0.87054 | 0.86625 | 0.87632 | 0.87309 | |||
| 68.0 | 0.88071 | 0.87241 | 0.86817 | 0.86387 | 0.87396 | 0.87071 | |||
| 69.0 | 0.87839 | 0.87004 | 0.86579 | 0.86148 | 0.87158 | 0.86833 | |||
| 70.0 | 0.87602 | 0.86766 | 0.86340 | 0.85908 | 0.86920 | 0.86593 | |||
| 71.0 | 0.87365 | 0.86527 | 0.86100 | 0.85667 | 0.86680 | 0.86352 | |||
| 71.9 | 78.3 | 0.86311 | –51.3 °C | ||||||
| 72.0 | 0.87127 | 0.86287 | 0.85859 | 0.85426 | 0.86440 | 0.86110 | |||
| 73.0 | 0.86888 | 0.86047 | 0.85618 | 0.85184 | 0.86200 | 0.85869 | |||
| 74.0 | 0.86648 | 0.85806 | 0.85376 | 0.84941 | 0.85958 | 0.85626 | |||
| 75.0 | 0.86408 | 0.85564 | 0.85135 | 0.84698 | 0.85716 | 0.85383 | |||
| 76.0 | 0.86168 | 0.85322 | 0.84891 | 0.84455 | 0.85473 | 0.85140 | |||
| 77.0 | 0.85927 | 0.85079 | 0.84647 | 0.84211 | 0.85230 | 0.84895 | |||
| 78.0 | 0.85685 | 0.84835 | 0.84403 | 0.83966 | 0.84985 | 0.84650 | |||
| 79.0 | 0.85422 | 0.84590 | 0.84158 | 0.83720 | 0.84740 | 0.84404 | |||
| 80.0 | 0.85197 | 0.84344 | 0.83911 | 0.83473 | 0.84494 | 0.84157 | |||
| 81.0 | 0.84950 | 0.84096 | 0.83664 | 0.83224 | 0.84245 | 0.83909 | |||
| 82.0 | 0.84702 | 0.83848 | 0.83415 | 0.82974 | 0.83997 | 0.83659 | |||
| 83.0 | 0.84453 | 0.83599 | 0.83164 | 0.82724 | 0.83747 | 0.83408 | |||
| 84.0 | 0.84203 | 0.83348 | 0.82913 | 0.82473 | 0.83496 | 0.83156 | |||
| 85.0 | 0.83951 | 0.83095 | 0.82660 | 0.82220 | 0.83242 | 0.82902 | |||
| 86.0 | 0.83697 | 0.82840 | 0.82405 | 0.81965 | 0.82987 | 0.82646 | |||
| 87.0 | 0.83441 | 0.82554 | 0.82148 | 0.81708 | 0.82729 | 0.82389 | |||
| 88.0 | 0.83181 | 0.82323 | 0.81888 | 0.81448 | 0.82469 | 0.82128 | |||
| 89.0 | 0.82919 | 0.82062 | 0.81626 | 0.81186 | 0.82207 | 0.81865 | |||
| 90.0 | 0.82654 | 0.81797 | 0.81362 | 0.80922 | 0.81942 | 0.81600 | |||
| 91.00 | 94.00 | 74.62 | 0.82386 | 0.81529 | 0.81094 | 0.80655 | 0.81674 | 0.81331 | |
| 92.0 | 0.82114 | 0.81257 | 0.80823 | 0.80384 | 0.81401 | 0.81060 | |||
| 93.0 | 0.81839 | 0.80983 | 0.80549 | 0.80111 | 0.81127 | 0.80785 | |||
| 94.0 | 0.81561 | 0.80705 | 0.80272 | 0.79835 | 0.80848 | 0.80507 | |||
| 95.0 | 0.81278 | 0.80424 | 0.79991 | 0.79555 | 0.80567 | 0.80225 | |||
| 96.0 | 0.80991 | 0.80138 | 0.79706 | 0.79271 | 0.80280 | 0.79939 | |||
| 97.0 | 0.80698 | 0.79846 | 0.79415 | 0.78981 | 0.79988 | 0.79648 | |||
| 98.0 | 0.80399 | 0.79547 | 0.79117 | 0.78684 | 0.79688 | 0.79349 | |||
| 99.0 | 0.80094 | 0.79243 | 0.78814 | 0.78382 | 0.79383 | 0.79045 | |||
| 100.0 | 100.0 | 79.39 | 0.79784 | 0.78934 | 0.78506 | 0.78075 | 0.79074 | 0.78736 | −114.3 °C |
| % wt ethanol | % vol ethanol | grams ethanol per 100 cc 15.56 °C | d 10 °C/4 °C | d 20 °C/4 °C | d 25 °C/4 °C | d 30 °C/4 °C | d 20 °C/20 °C | d 25 °C/25 °C | freezing temp. |
Boiling points of aqueous solutions
Data obtained from CRC Handbook of Chemistry 44th ed., p2391
| BP °C | Weight % ethanol | BP °C | Weight % ethanol | |||
| liquid | vapor | liquid | vapor | |||
| 78.1 | 95.5‡ | 95.5‡ | ||||
| 78.2 | 91 | 92 | 86.5 | 18 | 71 | |
| 78.4 | 85 | 89 | 87.0 | 17 | 70 | |
| 78.6 | 82 | 88 | 87.5 | 16 | 69 | |
| 78.8 | 80 | 87 | 88.0 | 15 | 68 | |
| 79.0 | 78 | 86 | 88.5 | 13 | 67 | |
| 79.2 | 76 | 85 | 89.0 | 12 | 65 | |
| 79.4 | 74 | 85 | 89.5 | 11 | 63 | |
| 79.6 | 72 | 84 | 90.0 | 10 | 61 | |
| 79.8 | 69 | 84 | 90.5 | 10 | 59 | |
| 80.0 | 67 | 83 | 91.0 | 9 | 57 | |
| 80.2 | 64 | 83 | 91.5 | 8 | 55 | |
| 80.4 | 62 | 82 | 92.0 | 8 | 53 | |
| 80.6 | 59 | 82 | 92.5 | 7 | 51 | |
| 80.8 | 56 | 81 | 93.0 | 6 | 49 | |
| 81.0 | 53 | 81 | 93.5 | 6 | 46 | |
| 81.2 | 50 | 80 | 94.0 | 5 | 44 | |
| 81.4 | 47 | 80 | 94.5 | 5 | 42 | |
| 81.6 | 45 | 80 | 95.0 | 4 | 39 | |
| 81.8 | 43 | 79 | 95.5 | 4 | 36 | |
| 82.0 | 41 | 79 | 96.0 | 3 | 33 | |
| 82.5 | 36 | 78 | 96.5 | 3 | 30 | |
| 83.0 | 33 | 78 | 97.0 | 2 | 27 | |
| 83.5 | 30 | 77 | 97.5 | 2 | 23 | |
| 84.0 | 27 | 77 | 98.0 | 1 | 19 | |
| 84.5 | 25 | 75 | 98.5 | 1 | 15 | |
| 85.0 | 23 | 74 | 99.0 | < 1 | 10 | |
| 85.5 | 21 | 73 | 99.5 | < 1 | 5 | |
| 86.0 | 20 | 72 | 100.0 | 0 | 0 | |
Charts
 |
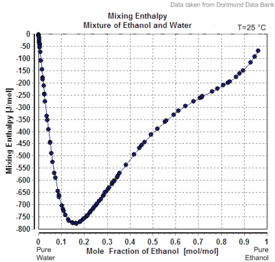 |
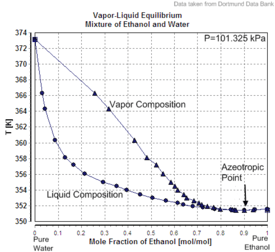 |
| Excess volume of the mixture of ethanol and water (volume contraction) | Heat of mixing of the mixture of ethanol and water | Vapor-liquid equilibrium of the mixture of ethanol and water (including azeotrope) |
 |
_Mixture_of_Ethanol_and_Dodecane.png) |
|
| Solid-liquid equilibrium of the mixture of ethanol and water (including eutecticum) | Miscibility gap in the mixture of dodecane and ethanol |
References
- ↑ "Ethanol – Compound Summary". The PubChem Project. USA: National Center for Biotechnology Information.
- 1 2 3 4 5 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.246. ISBN 1439855110.
- ↑ Ballinger, P.; Long, F.A. (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds1,2". Journal of the American Chemical Society. 82 (4): 795–798. doi:10.1021/ja01489a008.
- ↑ Arnett, E.M.; Venkatasubramaniam, K.G. (1983). "Thermochemical acidities in three superbase systems". J. Org. Chem. 48 (10): 1569–1578. doi:10.1021/jo00158a001.
- ↑ Lide, David R., ed. (2012). CRC Handbook of Chemistry and Physics (92 ed.). Boca Raton, FL.: CRC Press/Taylor and Francis. pp. 6–232.
- ↑ Lide, David R., ed. (2008). CRC Handbook of Chemistry and Physics (89 ed.). Boca Raton: CRC Press. pp. 9–55.
- ↑ Schmidt, Alexander (1974), Memorandum of Understanding Between The Bureau of Alcohol, Tobacco and Firearms and The Food and Drug Administration regarding the Promulgation and Enforcement of the Labeling Regulations Promulgated under the Federal Alcohol Administration Act, Washington, D.C.: Food and Drug Administration, retrieved 24 June 2015
- ↑ "Alcohol". Drugs.com. Retrieved 7 July 2015.
- ↑ WHO Expert Committee on Problems Related to Alcohol Consumption : second report. (PDF). Geneva, Switzerland: World Health Organization. 2007. p. 23. ISBN 9789241209441. Retrieved 3 March 2015.
...alcohol dependence (is) a substantial risk of regular heavy drinking...
- ↑ Vengeliene, V; Bilbao, A; Molander, A; Spanagel, R (May 2008). "Neuropharmacology of alcohol addiction". British Journal of Pharmacology. 154 (2): 299–315. doi:10.1038/bjp.2008.30. PMC 2442440
 . PMID 18311194.
. PMID 18311194. (Compulsive alcohol use) occurs only in a limited proportion of about 10–15% of alcohol users....
- ↑ Stogner, John M.; Eassey, John M.; Baldwin, Julie Marie; Miller, Bryan Lee (September 2014). "Innovative alcohol use: Assessing the prevalence of alcohol without liquid and other non-oral routes of alcohol administration". Drug and Alcohol Dependence. 142: 74–78. doi:10.1016/j.drugalcdep.2014.05.026. PMID 25012895.
- ↑ Gilman, Jodi M; Ramchandani, Vijay A; Crouss, Tess; Hommer, Daniel W (28 September 2011). "Subjective and Neural Responses to Intravenous Alcohol in Young Adults with Light and Heavy Drinking Patterns". Neuropsychopharmacology. 37 (2): 467–477. doi:10.1038/npp.2011.206. PMID 21956438.
- ↑ Swift, Robert (December 2003). "Direct measurement of alcohol and its metabolites". Addiction. 98: 73–80. doi:10.1046/j.1359-6357.2003.00605.x. PMID 14984244.
- 1 2 Becker, CE (12 August 2013). "The Clinical Pharmacology of Alcohol". California Medicine. 113 (3): 37–45. PMC 1501558
 . PMID 5457514.
. PMID 5457514. - ↑ "Ethanol Material Safety Data Sheet – Europe". Distill.com. Retrieved 2014-01-18.
- ↑ Sigma-Aldrich Co., Ethanol. Retrieved on 2015-02-05.
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0262". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "ChemIDplus – 64-17-5 – LFQSCWFLJHTTHZ-UHFFFAOYSA-N – Alcohol [USP] – Similar structures search, synonyms, formulas, resource links, and other chemical information.". Retrieved 13 October 2014.
- ↑ NMR-002: Sample Devices and Magnetic Susceptibility
- ↑ "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 12 May 2007.
- 1 2 Atkins, Peter (2010). Atkins' Physical Chemistry. Oxford University Press. pp. 913–947.
- 1 2 Lange's Handbook of Chemistry 10th ed, pp 1525-1528
- 1 2 CRC Handbook of Chemistry and Physics 44th ed. pp 2582-2584
- ↑ Lange's Handbook of Chemistry 10th ed, pp 1522-1524
- ↑ "Spectral Database for Organic Compounds" (Queriable database). Advanced Industrial Science and Technology. Retrieved 9 June 2007.
- Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov
This article is issued from Wikipedia - version of the 11/18/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.