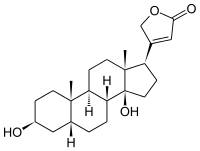
Digitoxigenin
 | |
| Names | |
|---|---|
| Other names
3-β,14-dihydroxy-5-β,14-β-card-20(22)-enolide 5β-Card-20(22)-enolide, 3β,14-dihydroxy- Card-20(22)-enolide, 3,14-dihydroxy-, (3β,5β)- NSC 407806 Δ20:22-3,14,21-Trihydroxynorcholenic acid lactone cerberigenin echujetin evonogenin thevetigenin | |
| Identifiers | |
| 143-62-4 | |
| 3D model (Jmol) | Interactive image |
| 95448 | |
| ChEMBL | ChEMBL1453 |
| ChemSpider | 3571902 |
| ECHA InfoCard | 100.005.095 |
| EC Number | 205-603-4 |
| PubChem | 4369270 |
| RTECS number | FH4975000 |
| |
| |
| Properties | |
| C23H34O4 | |
| Molar mass | 374.51 |
| Appearance | solid |
| Melting point | 252 to 253 °C (486 to 487 °F; 525 to 526 K) |
| Hazards | |
| Main hazards | Toxic |
| R-phrases | Toxic if swallowed |
| S-phrases | In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Digitoxigenin is cardenolide, obtained especially by hydrolysis of digitoxin[2] (refluxing digitoxin in a mixture of water, alcohol, and hydrochloric acid[3]), which is the aglycon of digitoxin.[4]
Digitoxigenin is used to prepare a compound called actodigin.[5]
12-Dehydrodigoxigenin is a related compound with the same structure, but featuring a ketone grouping at C-12.[6] In Lednicer's book on steroids, it is made from deoxycholic acid.
References
- ↑ http://www.sigmaaldrich.com/catalog/ProductDetail.do?N4=D9404|SIGMA&N5=SEARCH_CONCAT_PNO|BRAND_KEY&F=SPEC
- ↑ http://dictionary.reference.com/browse/digitoxigenin
- ↑ http://www.wrongdiagnosis.com/medical/digitoxigenin.htm
- ↑ http://www.mondofacto.com/facts/dictionary?digitoxigenin
- ↑ Ferland, J. M. (1974). "Synthetic Cardenolides and Related Products. III. Isocardenolides". Canadian Journal of Chemistry. 52 (9): 1652–1661. doi:10.1139/v74-239. ISSN 0008-4042.
- ↑ https://chem.nlm.nih.gov/chemidplus/rn/2842-85-5
This article is issued from Wikipedia - version of the 10/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.