Dichloromethane (data page)
Please find below supplementary chemical data about dichloromethane.
MSDS sheets
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction,[1] nD | 1.4242 |
| Abbe number | ? |
| Dielectric constant,[2] εr | 9.08 ε0 at 20°C |
| Dipole moment,[3] | 1.62 D |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[4] | 26.52 dyn/cm at 20°C |
| Viscosity[5] | 0.449 mPa·s at 15°C 0.393 mPa·s at 30°C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | ? K (? °C), ? Pa |
| Critical point[6] | 510 K (237 °C), 6100 kPa |
| Std enthalpy change of fusion, ΔfusH |
+6.160 kJ/mol |
| Std entropy change of fusion, ΔfusS |
? J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
+28.6 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−124.3 kJ/mol |
| Standard molar entropy, S |
174.5 J/(mol K) |
| Heat capacity, cp | 102.3 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−95.52 kJ/mol |
| Standard molar entropy, S |
270.28 J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| van der Waals' constants[7] | a = 1244 L2 kPa/mol2 b = 0.08689 liter per mole |
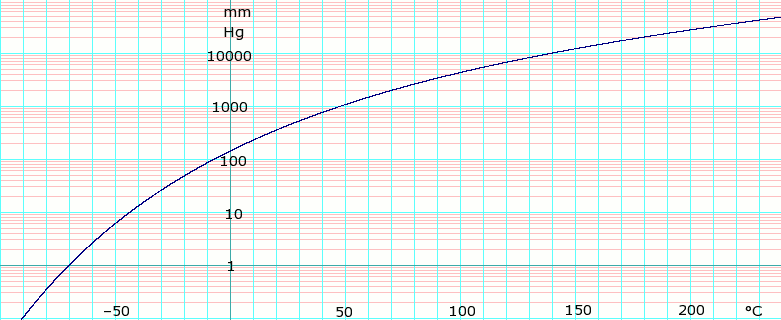
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | –70.0 | –43.3 | –22.3 | –6.3 | 24.1 | 40.7 | |
Table data obtained from CRC Handbook of Chemistry and Physics 47th ed.
Spectral data
| UV-Vis | |
|---|---|
| Spectrum | ? |
| Lambda-max | ? nm |
| Log Ε | ? |
| IR | |
| Spectrum | NIST |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | δ CDCl3 5.30 (s, 2H) |
| Carbon-13 NMR | δ CDCl3 53.5 |
| Other NMR data | ? |
| MS | |
| Masses of main fragments |
? |
| Except where noted otherwise, data are given for standard ambient temperature and pressure (25°C, 101.3 kPa) Disclaimer and references | |
Structure and properties data
| Structure and properties | |
|---|---|
| Index of refraction | 1.424 |
| Dielectric constant | 8.93 |
| Viscosity | 0.44 cP at 20 °C |
| Except where noted otherwise, data are given for standard ambient temperature and pressure (25°C, 101.3 kPa) Disclaimer and references | |
References
- ↑ "Methylene Chloride". chemicalland21.com. Retrieved 29 May 2007.
- ↑ CRC Handbook of Chemistry and Physics, 47th ed. p E-54
- ↑ The Microwave Spectra, Structure, Dipole Moment, and Chlorine Nuclear Quadrupole Coupling Constants of Methylene Chloride Rollie J. Myers and William D. Gwinn, J. Chem. Phys. 20, 1420 (1952); doi:10.1063/1.1700773
- ↑ CRC Handbook of Chemistry and Physics, 47th ed. pp F-28 - F-30
- ↑ CRC Handbook of Chemistry and Physics, 47th ed. pp F-33 - F-38
- 1 2 "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 29 May 2007.
- ↑ "van der Waal's Constants for Real Gases". Tom Stretton's Chemistry Pages. Retrieved 29 May 2007.
- NIST website
- G. W. C. Kaye and T. H. Laby, Tables of Physical & Chemical Constants at National Physical Laboratory
- Heat capacity
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
This article is issued from Wikipedia - version of the 8/24/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.