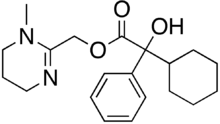
Oxyphencyclimine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | A03AA01 (WHO) |
| Identifiers | |
| |
| CAS Number |
125-53-1 |
| PubChem (CID) | 4642 |
| IUPHAR/BPS | 7256 |
| ChemSpider |
4481 |
| UNII |
4V44H1O8XI |
| KEGG |
D08325 |
| ChEMBL |
CHEMBL1200891 |
| ECHA InfoCard | 100.004.313 |
| Chemical and physical data | |
| Formula | C20H28N2O3 |
| Molar mass | 344.45 g/mol |
| |
| | |
Oxyphencyclimine is an antimuscarinic.
Synthesis
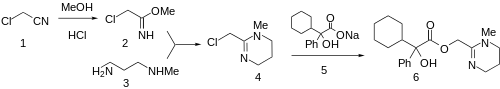
Reaction of chloroacetonitrile (1) with methanol and hydrogen chloride leads to the corresponding iminoether (Pinner reaction). Condensation of 2 with 3-methylaminopropylamine gives (3) gives the corresponding tetrahydropyrimidine (4). Displacement of the halogen with the sodium salt 5 affords oxyphencyclimine (6).
References
- ↑ Faust, J. A.; Mori, A.; Sahyun, M. (1959). "Antispasmodics: Esters of Heterocyclic Alcohols". Journal of the American Chemical Society. 81 (9): 2214. doi:10.1021/ja01518a051.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.