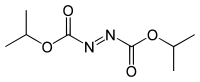
Diisopropyl azodicarboxylate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Diisopropyl azodicarboxylate | |
| Other names
DIAD | |
| Identifiers | |
| 2446-83-5 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 4515532 |
| ECHA InfoCard | 100.017.730 |
| PubChem | 5363146 |
| UNII | 7W701BXX4K |
| |
| |
| Properties | |
| C8H14N2O4 | |
| Molar mass | 202.21 g·mol−1 |
| Density | 1.027 g/cm3 |
| Melting point | 3 to 5 °C (37 to 41 °F; 276 to 278 K) |
| Boiling point | 75 °C (167 °F; 348 K) at 0.25 mmHg |
| insoluble | |
| Refractive index (nD) |
1.418-1.422 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| EU classification (DSD) |
Flammable (F) Irritant (Xi) Env. Danger (N) |
| R-phrases | R5, R11, R36, R37, R38, R43, R51, R53 |
| S-phrases | S16, S26, S29, S36, S37, S39, S47, S61 |
| Flash point | 106 °C (223 °F; 379 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diisopropyl azodicarboxylate (DIAD) is the diisopropyl ester of azodicarboxylic acid. It is used as a reagent in the production of many organic compounds. It is often used in the Mitsunobu reaction[1] where it serves as an oxidizer of triphenylphosphine to triphenylphosphine oxide. It has also been used to generate aza-Baylis-Hillman adducts with acrylates.[2] It can also serve as a selective deprotectant of N-benzyl groups in the presence of other protecting groups.[3]
It is sometimes preferred to diethyl azodicarboxylate (DEAD) because it is more hindered, and thus less likely to form hydrazide byproducts.
References
- ↑ "Fluka DIAD on Sigma-Aldrich". Retrieved 2008-11-18.
- ↑ Shi, Min; Zhao, Gui-Ling (2004). "Aza-Baylis–Hillman reactions of diisopropyl azodicarboxylate or diethyl azodicarboxylate with acrylates and acrylonitrile". Tetrahedron. 60 (9): 2083–2089. doi:10.1016/j.tet.2003.12.059.
- ↑ Kroutil, J.; Trnka, T.; Cerny, M. (2004). "Improved procedure for the selective N-debenzylation of benzylamines by diisopropyl azodicarboxylate". Synthesis. 3 (3): 446–450. doi:10.1055/s-2004-815937.
This article is issued from Wikipedia - version of the 10/13/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.