Cyclopentadiene
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclopenta-1,3-diene | |||
| Other names
1,3-Cyclopentadiene[1] Pyropentylene | |||
| Identifiers | |||
| 542-92-7 | |||
| 3D model (Jmol) | Interactive image | ||
| Abbreviations | CPD, HCp | ||
| 471171 | |||
| ChEBI | CHEBI:30664 | ||
| ChemSpider | 7330 | ||
| ECHA InfoCard | 100.008.033 | ||
| EC Number | 208-835-4 | ||
| 1311 | |||
| MeSH | 1,3-cyclopentadiene | ||
| PubChem | 7612 | ||
| RTECS number | GY1000000 | ||
| UNII | 5DFH9434HF | ||
| |||
| |||
| Properties | |||
| C5H6 | |||
| Molar mass | 66.10 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | irritating, terpene-like[1] | ||
| Density | 0.786 g cm−3 | ||
| Melting point | −90 °C; −130 °F; 183 K | ||
| Boiling point | 39 to 43 °C; 102 to 109 °F; 312 to 316 K | ||
| insoluble[1] | |||
| Vapor pressure | 400 mmHg (53 kPa)[1] | ||
| Acidity (pKa) | 16 | ||
| Basicity (pKb) | −2 | ||
| Structure | |||
| Planar[2] | |||
| Thermochemistry | |||
| 115.3 J K−1 mol−1 | |||
| Std molar entropy (S |
182.7 J K−1 mol−1 | ||
| Hazards | |||
| Flash point | 25 °C (77 °F; 298 K) | ||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) |
14,182 ppm (rat, 2 hr) 5091 ppm (mouse, 2 hr)[3] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 75 ppm (200 mg/m3)[1] | ||
| REL (Recommended) |
TWA 75 ppm (200 mg/m3)[1] | ||
| IDLH (Immediate danger) |
750 ppm[1] | ||
| Related compounds | |||
| Related hydrocarbons |
Benzene Cyclobutadiene Cyclopentene | ||
| Related compounds |
Dicyclopentadiene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction. This dimer can be restored by heating to give the monomer.
The compound is mainly used for the production of cyclopentene and its derivatives. It is popularly used as a precursor to the cyclopentadienyl ligand (Cp) in cyclopentadienyl complexes in organometallic chemistry.[4]
Production and reactions
Cyclopentadiene production is usually not distinguished from dicyclopentadiene since they are interconverted. They are obtained from coal tar (about 10–20 g/tonne) and by steam cracking of Naphtha (about 14 kg/tonne).[5] To obtain cyclopentadiene monomer, commercial dicyclopentadiene is cracked by heating to ~ 180 °C. The monomer is collected by distillation, and used soon thereafter.[6]
Sigmatropic rearrangement
The hydrogen atoms in cyclopentadiene undergo rapid [1,5]-sigmatropic shifts as indicated by 1H NMR spectra recorded at various temperatures.[7] Even more fluxional are the derivatives C5H5E(CH3)3 (E = Si, Ge, Sn), wherein the heavier element migrates from carbon to carbon with a low activation barrier.
Diels–Alder reactions
Famously, cyclopentadiene dimerizes via a reversible Diels–Alder reaction. The conversion occurs in hours at room temperature, but the monomer can be stored for days at −20 °C.[5] Cyclopentadiene is a highly reactive diene in the Diels–Alder reaction because less distortion of the diene termini is required to achieve the transition state geometry compared to other dienes.[8]
Deprotonation
The compound is unusually acidic (pKa = 16) for a hydrocarbon, a fact explained by the high stability of the aromatic cyclopentadienyl anion, C
5H−
5. Deprotonation can be achieved with a variety of bases, typically sodium hydride or even sodium metal. Simple compounds of this anion (such as the commercially available sodium cyclopentadienide) are often depicted as salts, although the free anion is not present in appreciable quantities in solution, more complex coordination compounds are known as cyclopentadienyl complexes.
Metallocene derivatives
Metallocenes and related cyclopentadienyl derivatives have been heavily investigated and represent a cornerstone of organometallic chemistry owing to their high stability. Indeed, the first metallocene characterised, ferrocene, was prepared the way many other metallocenes are prepared: by combining alkali metal derivatives of the form MC5H5 with dihalides of the transition metals:[10] As typical example, nickelocene forms upon treating nickel(II) chloride with sodium cyclopentadiene in THF.[11]
- NiCl2 + 2 NaC5H5 → Ni(C5H5)2 + 2 NaCl
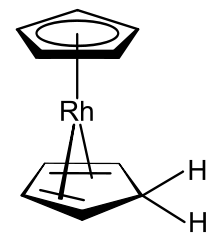
Organometallic complexes that include both the cyclopentadienyl anion and cyclopentadiene itself are known, one example of which is the rhodocene derivative produced from the rhodocene monomer in protic solvents.[12]
Uses
Cyclopentadiene is mainly useful as a precursor to cyclopentene and related monomers such as ethylidenenorbornene. Such species are used in the production of specialty polymers. Cyclopentadienyl complexes serve as reagents in organic synthesis. It was also used as the starting material in Leo Paquette's 1982 dodecahedrane synthesis.[13] The first step involved reductive dimerization of the molecule to give dihydrofulvalene, not simple addition to give dicyclopentadiene.

Abbreviation
The commonly used abbreviation of the cyclopentadienyl anion is Cp. The abbreviation played a part in the naming of copernicium: the original proposal for the element's symbol was also Cp, but because of the abbreviation for this anion and the fact that lutetium was originally named cassiopeium and had Cp for the symbol as well, the symbol for copernicium was changed to Cn.[14]
See also
- Aromaticity
- Methylcyclopentadiene, pentamethylcyclopentadiene and pentacyanocyclopentadiene, also found as ligands in organometallic chemistry
- Cyclopentadienone
References
- 1 2 3 4 5 6 7 "NIOSH Pocket Guide to Chemical Hazards #0170". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Faustov, Valery I.; Egorov, Mikhail P.; Nefedov, Oleg M.; Molin, Yuri N. (2000). "Ab initio G2 and DFT calculations on electron affinity of cyclopentadiene, silole, germole and their 2,3,4,5-tetraphenyl substituted analogs: structure, stability and EPR parameters of the radical anions". Phys. Chem. Chem. Phys. 2 (19): 4293–4297. doi:10.1039/b005247g.
- ↑ "Cyclopentadiene". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Hartwig, J. F. (2010). Organotransition Metal Chemistry: From Bonding to Catalysis. New York, NY: University Science Books. ISBN 1-891389-53-X.
- 1 2 Hönicke, Dieter; Födisch, Ringo; Claus, Peter; Olson, Michael (2005), "Cyclopentadiene and Cyclopentene", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH
- ↑ Moffett (1962). "Cyclopentadiene and 3-Chlorocyclopentene". Org. Synth.; Coll. Vol., 4, p. 238
- ↑ Streitwieser, A.; Heathcock, C. H.; Kosower, E. M. (1998). Introduction to Organic Chemistry (4th ed.). Upper Saddle River, NJ: Prentice Hall.
- ↑ Levandowski, Brian; Houk, Ken (2015). "Theoretical Analysis of Reactivity Patterns in Diels–Alder Reactions of Cyclopentadiene, Cyclohexadiene, and Cycloheptadiene with Symmetrical and Unsymmetrical Dienophiles.". J. Org. Chem. 80 (7): 3530–3537. doi:10.1021/acs.joc.5b00174.
- ↑ Fischer, E. O.; Wawersik, H. (1966). "Über Aromatenkomplexe von Metallen: LXXXVIII. Über Monomeres und Dimeres Dicyclopentadienylrhodium und Dicyclopentadienyliridium und Über Ein Neues Verfahren Zur Darstellung Ungeladener Metall-Aromaten-Komplexe" [On aromatic complexes of metals: LXXXVIII. On monomeric and dimeric dicyclopentadienylrhodium and dicyclopentadienyliridium, and a new method to represent uncharged metal–aromatic complexes]. J. Organomet. Chem. (in German). 5 (6): 559–567. doi:10.1016/S0022-328X(00)85160-8.
- ↑ Girolami, G. S.; Rauchfuss, T. B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. Mill Valley, CA: University Science Books. ISBN 0-935702-48-2.
- ↑ Jolly, W. L. (1970). The Synthesis and Characterization of Inorganic Compounds. Englewood Cliffs, NJ: Prentice-Hall. ISBN 0-13-879932-6.
- ↑ Kolle, U.; Grub, J. (1985). "Permethylmetallocene: 5. Reactions of Decamethylruthenium Cations". J. Organomet. Chem. 289 (1): 133–139. doi:10.1016/0022-328X(85)88034-7.
- ↑ Paquette, L. A.; Wyvratt, M. J. (1974). "Domino Diels–Alder reactions. I. Applications to the rapid construction of polyfused cyclopentanoid systems". J. Am. Chem. Soc. 96 (14): 4671–4673. doi:10.1021/ja00821a052.
- ↑ "Copernicium Video – The Periodic Table of Videos – University of Nottingham". Retrieved 2011-02-22.