Copper monosulfide
| | |
| Names | |
|---|---|
| IUPAC name
Copper sulfide | |
| Other names | |
| Identifiers | |
| 1317-40-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 14145 |
| ECHA InfoCard | 100.013.884 |
| PubChem | 14831 |
| RTECS number | GL8912000 |
| |
| |
| Properties | |
| CuS | |
| Molar mass | 95.611 g/mol |
| Appearance | black powder or lumps |
| Density | 4.76 g/cm3 |
| Melting point | above 500 °C (932 °F; 773 K) (decomposes)[1] |
| 0.000033 g/100 mL (18 °C) | |
| Solubility product (Ksp) |
6 x 10−37[2] |
| Solubility | soluble in HNO3, NH4OH, KCN insoluble in HCl, H2SO4 |
| Refractive index (nD) |
1.45 |
| Structure | |
| hexagonal | |
| Hazards | |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 1 mg/m3 (as Cu)[3] |
| REL (Recommended) |
TWA 1 mg/m3 (as Cu)[3] |
| IDLH (Immediate danger) |
TWA 100 mg/m3 (as Cu)[3] |
| Related compounds | |
| Other anions |
Copper(II) oxide |
| Other cations |
zinc sulfide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Copper sulfide is a chemical compound of copper and sulfur. It occurs in nature as the dark indigo blue mineral covellite. It is a moderate conductor of electricity.[4] A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts.[5] It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis[6] and photovoltaics.[7]
Manufacturing
Copper sulfide can be prepared by passing hydrogen sulfide gas into a solution of copper salt.
Alternatively, it can be prepared by melting an excess of sulfur with copper(I) sulfide or by precipitation with hydrogen sulfide from a solution of anhydrous copper(II) chloride in anhydrous ethanol.
The reaction of copper with molten sulfur followed by boiling sodium hydroxide and the reaction of sodium sulfide with aqueous copper sulfate will also produce copper sulfide.
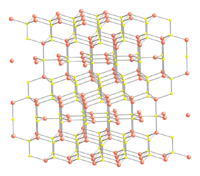
CuS structure and bonding
Copper sulfide crystallizes in the hexagonal crystal system, and this is the form of the mineral covellite. There is also an amorphous high pressure form [8] which on the basis of the Raman spectrum has been described as having a distorted covellite structure. An amorphous room temperature semiconducting form produced by the reaction of a Cu(II) ethylenediamine complex with thiourea has been reported, which transforms to the crystalline covellite form at 30 °C.[9]
The crystal structure of covellite has been reported several times,[10][11][12] and whilst these studies are in general agreement on assigning the space group P63/mmc there are small discrepancies in bond lengths and angles between them. The structure was described as "extraordinary" by Wells[13] and is quite different from copper(II) oxide, but similar to CuSe (klockmannite). The covellite unit cell contains 6 formula units (12 atoms)in which:
- 4 Cu atoms have tetrahedral coordination (see illustration).
- 2 Cu atoms have trigonal planar coordination (see illustration).
- 2 pairs of S atoms are only 207.1 pm apart [12] indicating the existence of an S-S bond (a disulfide unit).
- the 2 remaining S atoms form trigonal planar triangles around the copper atoms, and are surrounded by five Cu atoms in a pentagonal bipyramid (see illustration).
- The S atoms at each end of a disulfide unit are tetrahedrally coordinated to 3 tetrahedrally coordinated Cu atoms and the other S atom in the disulfide unit (see illustration).
The formulation of copper sulfide as CuIIS (i.e. containing no sulfur-sulfur bond) is clearly incompatible with the crystal structure, and also at variance with the observed diamagnetism [14] as a Cu(II) compound would have a d9 configuration and be expected to be paramagnetic.[5]
Studies using XPS[15][16][17][18] indicate that all of the copper atoms have an oxidation state of +1. This contradicts a formulation based on the crystal structure and obeying the octet rule that is found in many textbooks (e.g.[5][19]) describing CuS as containing both CuI and CuII i.e. (Cu+)2Cu2+(S2)2−S2−. An alternative formulation as (Cu+)3(S2−)(S2)− was proposed and supported by calculations.[20]
The formulation should not be interpreted as containing radical anion, but rather that there is a delocalized valence "hole".[20][21]
Electron paramagnetic resonance studies on the precipitation of Cu(II) salts indicates that the reduction of Cu(II) to Cu(I) occurs in solution.[22]
 |
 | 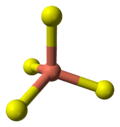 | 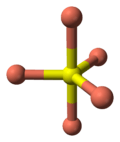 | 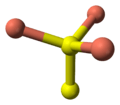 |
the crystal structure of covellite | coordination of copper | coordination of copper | coordination of sulfur | coordination of sulfur-note disulfide unit |
See also
- Copper sulfide for an overview of all copper sulfide phases
- copper(I) sulfide, Cu2S
- Covellite
References
- ↑ Blachnik, R.; Müller, A. (2000). "The formation of Cu2S from the elements I. Copper used in form of powders". Thermochimica Acta. 361 (1–2): 31–52. doi:10.1016/S0040-6031(00)00545-1.
- ↑ Rollie J. Myers (1986). "The new low value for the second dissociation constant for H2S: Its history, its best value, and its impact on the teaching of sulfide equilibria". J. Chem. Educ. 63: 687. doi:10.1021/ed063p687.
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0150". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Wells A.F. (1962) Structural Inorganic Chemistry 3d edition Oxford University Press
- 1 2 3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Kuchmii, S.Y.; Korzhak A.V.; Raevskaya A.E.; Kryukov A.I. (2001). "Catalysis of the Sodium Sulfide Reduction of Methylviologene by CuS Nanoparticles". Theoretical and Experimental Chemistry. New York: Springer. 37 (1): 36–41. doi:10.1023/A:1010465823376.
- ↑ Mane, R.S.; Lokhande C.D. (June 2000). "Chemical deposition method for metal chalcogenide thin films". Materials Chemistry and Physics. 65 (1): 1–31. doi:10.1016/S0254-0584(00)00217-0.
- ↑ Peiris, M; Sweeney, J.S.; Campbell, A.J.; Heinz D. L. (1996). "Pressure-induced amorphization of covellite, CuS". J. Chem. Phys. 104 (1): 11–16. Bibcode:1996JChPh.104...11P. doi:10.1063/1.470870.
- ↑ Grijalva, H.; Inoue, M.; Boggavarapu, S.; Calvert, P. (1996). "Amorphous and crystalline copper sulfides, CuS". J. Mater. Chem. 6 (7): 1157–1160. doi:10.1039/JM9960601157.
- ↑ Oftedal, I. (1932). Z. Kristallogr. 83: 9–25. Missing or empty
|title=(help) - ↑ Berry, L. G. (1954). "The crystal structure of covellite CuS and klockmannite CuSe". American Mineralogist. 39: 504.
- 1 2 Evans, H.T. Jr.; Konnert J. (1976). "Crystal structure refinement of covellite". American Mineralogist. 61: 996–1000.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ Magnetic susceptibility of the elements and inorganic compounds
- ↑ Nakai, I.; Sugitani, Y.; Nagashima, K.; Niwa, Y. (1978). "X-ray photoelectron spectroscopic study of copper minerals". Journal of Inorganic and Nuclear Chemistry. 40 (5): 789–791. doi:10.1016/0022-1902(78)80152-3.
- ↑ Folmer, J.C.W.; Jellinek F. (1980). "The valence of copper in sulfides and selenides: An X-ray photoelectron spectroscopy study". Journal of the Less Common Metals. 76 (1-2): 789–791. doi:10.1016/0022-5088(80)90019-3.
- ↑ Folmer, J.C.W.; Jellinek F.; Calis G.H.M (1988). "The electronic structure of pyrites, particularly CuS2 and Fe1−xCuxSe2: An XPS and Mössbauer study". Journal of Solid State Chemistry. 72 (1): 137–144. Bibcode:1988JSSCh..72..137F. doi:10.1016/0022-4596(88)90017-5.
- ↑ Goh, S.W.; Buckley A.N.; Lamb R.N. (February 2006). "Copper(II) sulfide?". Minerals Engineering. 19 (2): 204–208. doi:10.1016/j.mineng.2005.09.003.
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- 1 2 Liang, W.; Whangbo M, -H (February 1993). "Conductivity anisotropy and structural phase transition in Covellite CuS". Solid State Communications. 85 (5): 405–408. Bibcode:1993SSCom..85..405L. doi:10.1016/0038-1098(93)90689-K.
- ↑ Nozaki, H; Shibata, K; Ohhashi,N. (April 1991). "Metallic hole conduction in CuS". Journal of Solid State Chemistry. 91 (2): 306–311. Bibcode:1991JSSCh..91..306N. doi:10.1016/0022-4596(91)90085-V.
- ↑ Luther, GW; Theberge SM; Rozan TF; Rickard D; Rowlands CC; Oldroyd A. (February 2002). "Aqueous copper sulfide clusters as intermediates during copper sulfide formation.". Environ. Sci. Technol. 36 (3): 394–402. Bibcode:2002EnST...36..394L. doi:10.1021/es010906k. PMID 11871554.