Chalcanthite
| Chalcanthite | |
|---|---|
 | |
| General | |
| Category | Sulfate minerals |
| Formula (repeating unit) | CuSO4·5H2O |
| Strunz classification | 7.CB.20 |
| Crystal system | Triclinic |
| Crystal class |
Pinacoidal (1) H-M symbol: (1) |
| Space group | P1 |
| Unit cell |
a = 6.11 Å, b = 10.673 Å, c = 5.95 Å; α = 97.58°, β = 107.17°, γ = 77.55°; Z = 2 |
| Identification | |
| Color | Berlin blue to sky-blue, greenish blue |
| Crystal habit | Typically stalactitic, encrusted, reniform or massive. Natural crystals are rare, but are short prismatic or tabular |
| Twinning | Rare as cruciform twins |
| Cleavage | Perfect on {110}; interrupted on {110} |
| Fracture | Conchoidal |
| Mohs scale hardness | 2.5 |
| Luster | Vitreous |
| Streak | White |
| Diaphaneity | Transparent to translucent |
| Specific gravity | 2.12 - 2.3 |
| Optical properties | Biaxial (-) |
| Refractive index | nα = 1.514 nβ = 1.537 nγ = 1.543 |
| Birefringence | δ = 0.029 |
| Solubility | Soluble in water, turning it blue |
| Other characteristics |
|
| References | [1][2][3] |
Chalcanthite, whose name derives from the Greek, chalkos and anthos, meaning copper flower, is a richly-colored blue/green water-soluble sulfate mineral CuSO4·5H2O. It is commonly found in the late-stage oxidation zones of copper deposits. Due to its ready solubility, chalcanthite is more common in arid regions.
Chalcanthite is a pentahydrate and the most common member of a group of similar hydrated sulfates, the chalcanthite group. These other sulfates are identical in chemical composition to chalcanthite, with the exception of replacement of the copper ion by either manganese as jokokuite, iron as siderotil, or magnesium as pentahydrite.[4]
Other names include blue stone, blue vitriol, and copper vitriol.[2]
Uses of chalcanthite

As chalcanthite is a copper mineral, it can be used as an ore of copper. However, its ready solubility in water means that it tends to crystallize, dissolve, and recrystallize as crusts over any mine surface in more humid regions. Therefore, chalcanthite is only found in the most arid regions in sufficiently large quantities for use as an ore.
Secondarily, chalcanthite, due to its rich color and beautiful crystals, is a sought after collector's mineral. However, as with its viability as an ore, the solubility of the mineral causes significant problems. First, the mineral readily absorbs and releases its water content, which, over time, leads to a disintegration of the crystal structure, destroying even the finest specimens. It is critical to store specimens properly to limit exposure to humidity. Second, higher quality crystals can be easily grown synthetically, and, as such, there is a concern that disreputable mineral dealers would present a sample as natural when it is not.
Associated minerals
Given that chalcanthite is found in oxidized copper deposits, it is frequently found in association with other copper minerals. Frequently associated minerals include:
- Calcite and its polymorph, aragonite, both CaCO3
- Brochantite, Cu4(SO4)(OH)6
- Chalcopyrite, CuFeS2
- Malachite, Cu2(CO3)(OH)2
- Melanterite, FeSO4 · 7H2O
Notes for identification
Chalcanthite's blue color is one of its most notable features, but it is insufficient in identification. Other useful tests include associated minerals, crystal habit, solubility and subsequent coloring of the water blue, and taste. A special note on tasting chalcanthite, however: chalcanthite has a sweetly metallic taste, but taste testing should not be done haphazardly. The specimen should be never touched with the tongue, as chalcanthite is poisonous. The liquid from chalcanthite will also stain skin blue for several days.
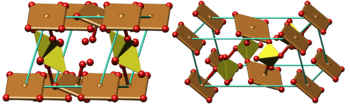
Color code: :Copper, Cu: brown :Sulfur, S: olive :Oxygen, O: red :Cell: cyan
See also
References
| Wikimedia Commons has media related to Category:Chalcanthite. |