Bromocresol purple
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4,4'-(1,1-Dioxido-3H-2,1-benzoxathiole-3,3-diyl)-bis(2-bromo-6-methylphenol) | |
| Other names
5′,5″-Dibromo-o-cresolsulfonephthalein Bromcresol purple | |
| Identifiers | |
| 115-40-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:86154 |
| ChemSpider | 7974 |
| ECHA InfoCard | 100.003.716 |
| PubChem | 8273 |
| |
| |
| Properties | |
| C21H16Br2O5S | |
| Molar mass | 540.22 g·mol−1 |
| Appearance | Purple powder |
| Melting point | 241 to 242 °C (466 to 468 °F; 514 to 515 K) (decomposition) |
| < 0.1 % | |
| Hazards | |
| R-phrases | R36/37/38 |
| S-phrases | S26, S36 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
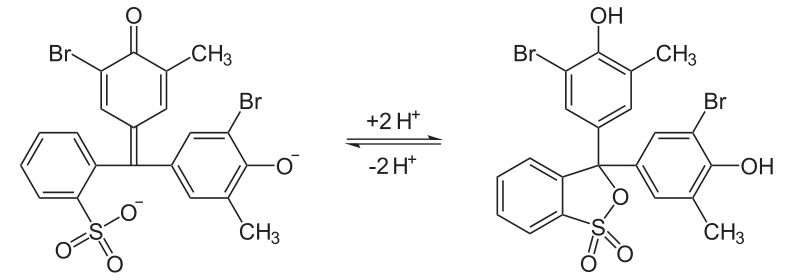
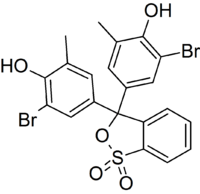
Bromocresol purple (BCP) or 5′,5″-dibromo-o-cresolsulfophthalein, is a dye of the triphenylmethane family (triarylmethane dyes) and a pH indicator. It is colored yellow below pH 5.2, and violet above pH 6.8. In its cyclic sulfonate ester form, it has a pKa value of 6.3, and is usually prepared as a 0.04% aqueous solution.[1]
Uses
| Bromocresol purple (pH indicator) | ||
| below pH 5.2 | above pH 6.8 | |
| 5.2 | ⇌ | 6.8 |
Bromocresol purple is used in medical laboratories to measure albumin, although it is no better in this role than the older method using bromocresol green.[2] In microbiology, it is used for staining dead cells based on their acidity, and for the isolation and assaying of lactic acid bacteria.[3][4]
In photographic processing, it can be used as an additive to acid stop baths to indicate that the bath has reached neutral pH and needs to be replaced.[5]
Bromocresol purple milk solids glucose agar is used as a medium used to distinguish dermatophytes from bacteria and other organisms in cases of ringworm fungus (T. verrucosum) infestation in cattle and other animals.[6][7]
pH Indicator
Similar to bromocresol green, the structure of bromocresol purple changes with pH. The low pH (acidic) form is yellow in solution and the high pH (basic) form is purple; the sultone (cyclic sulfonic ester) is the acidic form in the following equilibrium.
References
- ↑ "Bromocresol Purple". NCBI PubChem. National Center for Biotechnology Information.
- ↑ McGinlay, J.M.; Payne, R.B. (July 1988). "Serum albumin by dye-binding: bromocresol green or bromocresol purple? The case for conservatism." (PDF). Annals of Clinical Biochemistry. 25 (4): 417–421. doi:10.1177/000456328802500417. PMID 3214125.
- ↑ Kurzweilová, H.; Sigler, K. (November 1993). "Fluorescent staining with bromocresol purple: a rapid method for determining yeast cell dead count developed as an assay of killer toxin activity.". Yeast. 9 (11): 1207–1211. doi:10.1002/yea.320091107. PMID 7509098.
- ↑ Lee, H.M.; Lee, Y. (June 2008). "A differential medium for lactic acid-producing bacteria in a mixed culture". Letters of Applied Microbiology. 46 (6): 676–681. doi:10.1111/j.1472-765X.2008.02371.x. PMID 18444977.

- ↑ Anchell, Steve (2016). The Darkroom Cookbook (4 ed.). Routledge. ISBN 9781317337607 – via Google Books.
- ↑ Kane, J.; Summerbell, R.; Sigler, L.; Krajden, S.; Land, G. (1997). Laboratory Handbook of Dermatophytes: A Clinical Guide and Laboratory Handbook of Dermatophytes and Other Filamentous Fungi from Skin, Hair, and Nails. Belmont, CA: Star Publishing Company. ISBN 9780898631579.
- ↑ Beneke, E. S.; Rogers, A. L. (1996). Medical Mycology and Human Mycoses (illustrated ed.). Belmont, CA: Star Publishing Company. pp. 85–90. ISBN 9780898631753.
External links
| Wikimedia Commons has media related to Bromocresol purple. |