Avidin
| Avidin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 core-streptavidin mutant d128a at pH 4.5 | |||||||||
| Identifiers | |||||||||
| Symbol | Avidin | ||||||||
| Pfam | PF01382 | ||||||||
| InterPro | IPR005468 | ||||||||
| PROSITE | PDOC00499 | ||||||||
| SCOP | 1slf | ||||||||
| SUPERFAMILY | 1slf | ||||||||
| |||||||||
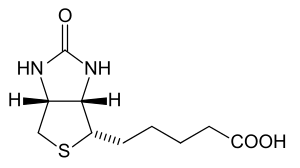
Avidin is a tetrameric biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. There are also dimeric members of the avidin family found in some bacteria.[1] In chicken egg white, avidin makes up approximately 0.05% of total protein (approximately 180 ug per egg). The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of avidin is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds.[2]
In its tetrameric form, avidin is estimated to be between 66–69 kDa in size.[3] 10% of the molecular weight is attributed to carbohydrate content composed of four to five mannose and three N-acetylglucosamine residues.[4] The carbohydrate moieties of avidin contain at least three unique oligosaccharide structural types that are similar in structure and composition.[5]
Functional avidin is found only in raw egg, as the biotin avidity of the protein is destroyed by cooking. The natural function of avidin in eggs is not known, although it has been postulated to be made in the oviduct as a bacterial growth-inhibitor, by binding biotin helpful for bacterial growth. As evidence for this, streptavidin, a loosely related protein with equal biotin affinity and a very similar binding site, is made by certain strains of Streptomyces bacteria, and is thought to serve to inhibit the growth of competing bacteria, in the manner of an antibiotic.[6]
A non-glycosylated form of avidin has been isolated from commercially prepared product; however, it is not conclusive as to whether the non-glycosylated form occurs naturally or is a product of the manufacturing process.[7]
Discovery of avidin

Avidin was first discovered by Esmond Emerson Snell (1914–2003). The route to discovery began with the observation that chicks on a diet of raw egg-white were deficient in biotin, despite availability of the vitamin in their diet.[8] It was concluded that a component of the egg-white was sequestering biotin[8] which Snell verified in vitro using a yeast assay.[9] Snell later isolated the component of egg white responsible for biotin binding, and, in collaboration with Paul Gyorgy, confirmed that the isolated egg protein was the cause of biotin deficiency or “egg white injury”.[10] At the time the protein had been tentatively named avidalbumin (literally, hungry albumin) by the involved researchers at the University of Texas.[10] The name of the protein was later revised to "avidin" based on its affinity for biotin (avid + biotin).[11]
Applications of avidin
Research in the 1970s helped establish the avidin-biotin system as a powerful tool in biological sciences. Aware of the strength and specificity of the avidin-biotin complex, researchers began to exploit avidin and streptavidin as probes and affinity matrices in numerous research projects.[12][13][14][15] Soon after, researchers Bayer and Wilchek developed new methods and reagents to biotinylate antibodies and other biomolecules,[16][17] allowing the transfer of the avidin-biotin system to a range of biotechnological applications. Today, avidin is used in applications ranging from research and diagnostics to medical devices and pharmaceuticals.
Avidin's affinity for biotin is exploited in wide-ranging biochemical assays, including western blot, ELISA, ELISPOT and pull-down assays. In some cases the use of biotinylated antibodies has allowed the replacement of radioiodine labeled antibodies in radioimmunoassay systems, to give an assay system which is not radioactive.
Avidin immobilized onto solid supports is also used as purification media to capture biotin-labelled protein or nucleic acid molecules. For example, cell surface proteins can be specifically labelled with membrane impermeable biotin reagent, then specifically captured using an avidin-based support.
Modified forms of avidin
As a basically charged glycoprotein, avidin exhibits non-specific binding in some applications. Neutravidin, a deglycosylated avidin with modified arginines, exhibits a more neutral isoelectric point (pI) and is available as an alternative to native avidin, whenever problems of non-specific binding arise. Deglycosylated, neutral forms of avidin are available through Sigma-Aldrich (Extravidin), Thermo Scientific (NeutrAvidin), Invitrogen (NeutrAvidin), and Belovo (NeutraLite).
Given the strength of the avidin-biotin bond, dissociation of the avidin-biotin complex requires extreme conditions that cause protein denaturation. The non-reversible nature of the avidin-biotin complex can limit avidin’s application in affinity chromatography applications where release of the captured ligand is desirable. Researchers have created an avidin with reversible binding characteristics through nitration or iodination of the binding site tyrosine.[18] The modified avidin exhibits strong biotin binding characteristics at pH 4 and releases biotin at a pH of 10 or higher.[18] A monomeric form of avidin with a reduced affinity for biotin is also employed in many commercially available affinity resins. The monomeric avidin is created by treatment of immobilized native avidin with urea or guanidine HCl (6–8 M), giving it a lower dissociation KD ≈ 10−7M.[19] This allows elution from the avidin matrix to occur under milder, non-denaturing conditions, using low concentrations of biotin or low pH conditions. For a single high affinity biotin binding site without crosslinking, a monovalent version of avidin's distant relative, streptavidin, may be used.[20]
Blocking of biotin binding
The thermal stability and biotin binding activity of avidin are of both practical and theoretical interest to researchers, as avidin's stability is unusually high and avidin is an antinutrient in human food.[21] A 1966 study published in Biochemical and Biophysical Research Communications found that the structure of avidin remains stable at temperatures below 70 °C (158 °F). Above 70 °C (158 °F), avidin's structure is rapidly disrupted and by 85 °C (185 °F), extensive loss of structure and ability to bind biotin is found.[22] A 1991 assay for the Journal of Food Science detected substantial avidin activity in cooked egg white: "mean residual avidin activity in fried, poached and boiled (2 min) egg white was 33, 71 and 40% of the activity in raw egg white." The assay surmised that cooking times were not sufficient to adequately heat all cold spot areas within the egg white. Complete inactivation of avidin's biotin binding capacity required boiling for over 4 minutes.[23]
A 1992 study found that thermal inactivation of the biotin binding activity of avidin was described by D121 °C = 25 min and z = 33 °C. The study disagreed with prior assumptions "that the binding site of avidin is destroyed on heat denaturation".[21]
The biotin-binding properties of avidin were exploited during the development of idrabiotaparinux, a long-acting low molecular weight heparin used in the treatment of venous thrombosis. Due to the long-acting nature of idraparinux, concerns were made about the clinical management of bleeding complications. By adding a biotin moiety to the idraparinux molecule, idrabiotaparinux was formed; its anticoagulant activity in the setting of a bleeding event can be reversed through an intravenous infusion of avidin.[24]
See also
Notes
- ↑ Nurminen et al. 2007
- ↑ Green 1963
- ↑ Korpela 1984
- ↑ Green 1975
- ↑ Bruch & White 1982
- ↑ Hendrickson et al. 1989
- ↑ Hiller et al. 1987
- 1 2 Eakin, McKinley & Williams 1940
- ↑ Snell, Eakin & Williams 1940
- 1 2 Gyorgy 1941
- ↑ Kresge, Simoni & Hill 2004
- ↑ Hofmann & Kiso 1976
- ↑ Bayer et al. 1976
- ↑ Angerer et al. 1976
- ↑ Heffegeness & Ash 1977
- ↑ Bayer, Zalis & Wilchek 1985
- ↑ Wilchek, Ben-Hur & Bayer 1986
- 1 2 Morag, Bayer & Wilchek 1996
- ↑ Kohanski & Lane 1990
- ↑ Howarth; et al. (2006). "A monovalent streptavidin with a single femtomolar biotin binding site". 3 (4). doi:10.1038/nmeth861. PMC 2576293
 . PMID 16554831.
. PMID 16554831. - 1 2 Durance & Wong 1992
- ↑ Pritchard, McCormick & Wright 1966
- ↑ Durance 1991
- ↑ Büller 2012
References
- Angerer, Lynne; Davidson, Norman; Murphy, William; Lynch, Dennis; Attardi, Giuseppe (1976). "An electron microscope study of the relative positions of the 4S and ribosomal RNA genes in HeLa cell mitochondrial DNA". Cell. 9 (1): 81–90. doi:10.1016/0092-8674(76)90054-4. PMID 975242.
- Büller, Harry; Gallus, Alex; Pillion, Gerard; Prins, Martin; Raskob, Gary (2012). "Enoxaparin followed by once-weekly idrabiotaparinux versus enoxaparin plus warfarin for patients with acute symptomatic pulmonary embolism: a randomised, double-blind, double-dummy, non-inferiority trial". Lancet. 379 (9811): 123–129. doi:10.1016/S0140-6736(11)61505-5. PMID 22130488.
- Bayer, Edward A.; Zalis, Mariano G.; Wilchek, Meir (1985). "3-(N-maleimido-propionyl) biocytin: A versatile thiol-specific biotinylating reagent". Analytical Biochemistry. 149 (2): 529–36. doi:10.1016/0003-2697(85)90609-8. PMID 3935007.
- Bayer, E. A.; Skutelsky, E.; Wynne, D.; Wilchek, M. (1976). "Preparation of ferritin-avidin conjugates by reductive alkylation for use in electron microscopic cytochemistry". Journal of Histochemistry & Cytochemistry. 24 (8): 933–9. doi:10.1177/24.8.182877. PMID 182877.
- Bruch, Richard C.; White, Harold B. (1982). "Compositional and structural heterogeneity of avidin glycopeptides". Biochemistry. 21 (21): 5334–41. doi:10.1021/bi00264a033. PMID 6816268.
- Durance, T. D. (1991). "Residual Avid in Activity in Cooked Egg White Assayed with Improved Sensitivity". Journal of Food Science. 56 (3): 707–9. doi:10.1111/j.1365-2621.1991.tb05361.x.
- Durance, T.D.; Wong, N.S. (1992). "Kinetics of thermal inactivation of avidin". Food Research International. 25 (2): 89–92. doi:10.1016/0963-9969(92)90148-X.
- Eakin, R. E.; McKinley, W. A.; Williams, R. J. (1940). "Egg-White Injury in Chicks and Its Relationship to a Deficiency of Vitamin H (Biotin)". Science. 92 (2384): 224–5. Bibcode:1940Sci....92..224E. doi:10.1126/science.92.2384.224. PMID 17743857.
- Green, NM (1963). "Avidin. 1. The Use of (14-C)Biotin for Kinetic Studies and for Assay" (PDF). The Biochemical Journal. 89: 585–91. PMC 1202466
 . PMID 14101979.
. PMID 14101979. - Green, N. Michael (1975). "Avidin". In Anfinsen, Christian B.; Edsall, John Tileston; Richards, Frederic Middlebrook. Advances in Protein Chemistry Volume. 29. pp. 85–133. doi:10.1016/S0065-3233(08)60411-8. ISBN 978-0-12-034229-7.
- Gyorgy, P.; Rose, C. S.; Eakin, R. E.; Snell, E. E.; Williams, R. J. (1941). "Egg-White Injury As the Result of Nonabsorption or Inactivation of Biotin". Science. 93 (2420): 477–8. Bibcode:1941Sci....93..477G. doi:10.1126/science.93.2420.477. PMID 17757050.
- Heggeness, Michael H.; Ash, John F. (1977). "Use of the avidin-biotin complex for the localization of actin and myosin with fluorescence microscopy". The Journal of Cell Biology. 73 (3): 783–8. doi:10.1083/jcb.73.3.783. PMC 2111432
 . PMID 326797.
. PMID 326797. - Nurminen, Kirsi P.; Helppolainen, Satu H.; Määttä, Juha A. E.; et al. (2007). "Rhizavidin from Rhizobium etli: The first natural dimer in the avidin protein family". Biochemical Journal. 405 (3): 397–405. doi:10.1042/BJ20070076. PMC 2267316
 . PMID 17447892.
. PMID 17447892. - Hendrickson, Wayne A.; Pahler, Arno; Smith, Janet L.; Satow, Yoshinori; Merritt, Ethan A.; Phizackerley, R. Paul (1989). "Crystal Structure of Core Streptavidin Determined from Multiwavelength Anomalous Diffraction of Synchrotron Radiation". Proceedings of the National Academy of Sciences. 86 (7): 2190–4. Bibcode:1989PNAS...86.2190H. doi:10.1073/pnas.86.7.2190. JSTOR 33443. PMC 286877
 . PMID 2928324.
. PMID 2928324. - Hiller, Y; Gershoni, JM; Bayer, EA; Wilchek, M (1987). "Biotin binding to avidin. Oligosaccharide side chain not required for ligand association". The Biochemical Journal. 248 (1): 167–71. PMC 1148514
 . PMID 3435435.
. PMID 3435435. - Hofmann, Klaus; Kiso, Yoshiaki; Kiso, Y (1976). "An Approach to the Targeted Attachment of Peptides and Proteins to Solid Supports". Proceedings of the National Academy of Sciences. 73 (10): 1784–5. Bibcode:1976PNAS...73.3516H. doi:10.1073/pnas.73.10.3516. JSTOR 66631. PMID 18051425.
- Kohanski, Ronald A.; Daniel Lane, M. (1990). "Monovalent avidin affinity columns". Avidin-Biotin Technology. Methods in Enzymology. 184. pp. 194–200. doi:10.1016/0076-6879(90)84274-K. ISBN 978-0-12-182085-5. PMID 2388570.
- Korpela, J (1984). "Avidin, a high affinity biotin-binding protein, as a tool and subject of biological research". Medical Biology. 62 (1): 5–26. PMID 6379329.
- Kresge, Nicole; Simoni, Robert D.; Hill, Robert L. (2004). "The Discovery of Avidin by Esmond E. Snell". The Journal of Biological Chemistry. 279 (41): e5.
- Morag, Ely; Bayer, Edward A.; Wilchek, Meir (1996). "Reversibility of biotin-binding by selective modification of tyrosine in avidin". The Biochemical Journal. 316 (1): 193–9. PMC 1217322
 . PMID 8645205.
. PMID 8645205. - Pritchard, Alan B.; McCormick, Donald B.; Wright, Lemuel D. (1966). "Optical rotatory dispersion studies of the heat denaturation of avidin and the avidin-biotin complex". Biochemical and Biophysical Research Communications. 25 (5): 524–8. doi:10.1016/0006-291X(66)90623-1.
- Snell, Esmond E.; Eakin, Robert E.; Williams, Roger J. (1940). "A Quantitative Test for Biotin and Observations Regarding its Occurrence and Properties". Journal of the American Chemical Society. 62: 175–8. doi:10.1021/ja01858a052.
- Wilchek, Meir; Ben-Hur, Haya; Bayer, Edward A. (1986). "P-Diazobenzoyl biocytin — A new biotinylating reagent for the labeling of tyrosines and histidines in proteins". Biochemical and Biophysical Research Communications. 138 (2): 872–9. doi:10.1016/S0006-291X(86)80577-0. PMID 3741438.
External links
- Egg-stremely useful interaction QUite Interesting PDB Structure article at PDBe