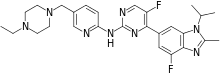
Abemaciclib
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| Synonyms | LY2835219 |
| CAS Number | 1231929-97-7 |
| PubChem (CID) | 46220502 |
| ChemSpider | 29340700 |
| ChEMBL | CHEMBL3301610 |
| PDB ligand ID | 6ZV (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C27H32F2N8 |
| Molar mass | 506.61 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
Abemaciclib (previously known as LY2835219) is a CDK inhibitor selective for CDK4 and CDK6.[1] It is an investigational drug for various types of cancer developed by Eli Lilly. It was designated as a breakthrough therapy by the U.S. Food and Drug Administration in October 2015.[2]
Clinical trials
Successful Phase I[3] and Phase II[4] trials against breast cancer were announced in May and December 2014 respectively.
As of early 2016 Abemaciclib is involved in 3 Phase III clinical trials:
- The JUNIPER Study is comparing Abemaciclib against Erlotinib in patients with stage IV Non-small-cell lung carcinoma[5]
- The MONARCH 2 study is investigating the effectiveness of Abemaciclib in combination with Fulvestrant for women with breast cancer.[6] It is due to end in Feb 2017.[7]
- The MONARCH 3 study is investigating the effectiveness of Abemaciclib, plus either anastrozole or letrozole, as a first-line treatment for women with breast cancer.[8] The trail is expected to end in June 2017.[9]
Chemistry
Abemaciclib may be synthesized in a 4 step manner using a Suzuki coupling, followed by a Buchwald–Hartwig amination with the final step being a reductive amination using the Leuckart reaction.[10]
References
- ↑ Lu, Janice (13 August 2015). "Palbociclib: a first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer". Journal of Hematology & Oncology. 8 (1). doi:10.1186/s13045-015-0194-5.
- ↑ "FDA's Breakthrough Therapy Designation to Abemaciclib for Breast Cancer". Oncology Times. LWW Journals. Retrieved 30 March 2016.
- ↑ LY2835219 Shows Strong Single-Agent Activity in Preliminary Study in Metastatic Breast Cancer
- ↑ Clinical Activity of Abemaciclib (LY2835219), a Cell Cycle Inhibitor Selective for CDK4 and CDK6, in Patients with Relapsed or Refractory Mantle Cell Lymphoma
- ↑ Goldman, Jonathan W.; Shi, Peipei; Reck, Martin; Paz-Ares, Luis; Koustenis, Andrew; Hurt, Karla C. (January 2016). "Treatment Rationale and Study Design for the JUNIPER Study: A Randomized Phase III Study of Abemaciclib With Best Supportive Care Versus Erlotinib With Best Supportive Care in Patients With Stage IV Non–Small-Cell Lung Cancer With a Detectable KRAS Mutation Whose Disease Has Progressed After Platinum-Based Chemotherapy". Clinical Lung Cancer. 17 (1): 80–84. doi:10.1016/j.cllc.2015.08.003.
- ↑ Llombart, Antonio; Toi, Masakazu; Klise, Suzanne R; Frenzel, Martin; Chan, Edward M; Sledge, George W (30 April 2015). "Abstract OT1-1-07: A phase III study of abemaciclib (LY2835219) combined with fulvestrant in women with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) breast cancer (MONARCH 2)". Cancer Research. 75 (9 Supplement): OT1–1–07–OT1–1–07. doi:10.1158/1538-7445.SABCS14-OT1-1-07.
- ↑ A Study of Abemaciclib (LY2835219) Combined With Fulvestrant in Women With Hormone Receptor Positive HER2 Negative Breast Cancer (MONARCH 2)
- ↑ Goetz, Matthew P; Toi, Masakazu (2015). "MONARCH 3: A randomized phase III study of anastrozole or letrozole plus abemaciclib, a CDK4/6 inhibitor, or placebo in first-line treatment of women with HR+, HER2-locoregionally recurrent or metastatic breast cancer (MBC).". Journal of Clinical Oncology. 33 (No 15_suppl).
- ↑ A Study of Nonsteroidal Aromatase Inhibitors Plus Abemaciclib (LY2835219) in Postmenopausal Women With Breast Cancer (MONARCH 3)
- ↑ Frederick, Michael O.; Kjell, Douglas P. (February 2015). "A synthesis of abemaciclib utilizing a Leuckart–Wallach reaction". Tetrahedron Letters. 56 (7): 949–951. doi:10.1016/j.tetlet.2014.12.082.
This article is issued from Wikipedia - version of the 8/24/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.