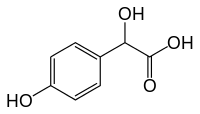
4-Hydroxymandelic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hydroxy(4-hydroxyphenyl)acetic acid | |
| Other names
2-Hydroxy-2-(4-hydroxyphenyl)acetic acid 4-Hydroxyphenylglycolic acid p-Hydroxymandelic acid 4-Hydroxymandelate | |
| Identifiers | |
| 1198-84-1 184901-84-6 (hydrate) | |
| 3D model (Jmol) | Interactive image |
| 2365374 | |
| ChemSpider | 321 |
| ECHA InfoCard | 100.013.490 |
| EC Number | 214-839-7 |
| PubChem | 328 |
| |
| |
| Properties | |
| C8H8O4 | |
| Molar mass | 168.15 g·mol−1 |
| Appearance | Light red powder |
| Melting point | 89 °C (192 °F; 362 K) |
| Hazards | |
| Safety data sheet | MSDS at Sigma Aldrich |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Hydroxymandelic acid is a chemical compound used to synthesize atenolol.[1] The compound typically occurs as a monohydrate (C8H8O4 • H2O at 186.16 g/mol).
Synthesis
4-Hydroxymandelic acid is obtained by the condensation reaction between phenol and glyoxylic acid.[1]
See also
References
- 1 2 Mattioda, Georges & Christidis, Yani (2000). "Glyoxylic Acid". Ullmann's Encyclopedia of Industrial Chemistry: pg. 2. doi:10.1002/14356007.a12_495. Retrieved 27 December 2013.
This article is issued from Wikipedia - version of the 9/10/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.